Translate this page into:
Dermatological Adverse Effects of Covid-19 Vaccine among Healthcare Workers: Findings from a Tertiary Care Hospital in Northern India
*Corresponding author: Vineet Relhan, Department of Dermatology, Maulana Azad Medical College and Associated Lok Nayak Hospital, New Delhi, Delhi, India. vineetrelhan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Choudhary H, Wason R, Goel M, Relhan V, Majhi MM. Dermatological Adverse Effects of Covid-19 Vaccine among Healthcare Workers: Findings from a Tertiary Care Hospital in Northern India. Indian J Postgrad Dermatol 2023;1:72-8.
Abstract
Objectives:
The objective of this study was to identify the cutaneous manifestations that developed after covid-19 vaccination, with Covaxin and Covishield, in healthcare workers (HCW) and to identify any associated factors.
Materials and Methods:
A cross-sectional study was conducted over 3 months using an online and offline questionnaire that was circulated among the HCW working at a tertiary care hospital in Delhi. All the vaccinated HCWs who gave consent (n = 676) (n = number of participants) were included in the study. Data were summarised in an excel sheet and then analysed using SPSS, version 25.
Results:
Out of the 676 vaccine recipients, a higher number of participants were vaccinated with Covishield compared to Covaxin. Only 14 (2.0%) subjects developed new adverse dermatological manifestations following either vaccination. A higher incidence of adverse reaction was observed with Covaxin than with Covishield, but the difference was not significant. A significantly higher number of vaccinated paramedical workers developed new dermatological manifestations in the study period [P-value (probability value)] (P = 0.018).
Conclusion:
Our study demonstrated a low rate of cutaneous adverse reactions following vaccination with either Covaxin or Covishield. Further studies are hence needed to corroborate our findings.
Keywords
Covid-19 vaccines
Healthcare workers
Adverse effects
INTRODUCTION
India kick–started its drive for covid-19 vaccination on 16 January, 2021. Healthcare workers (HCW) and other frontline workers were among the first beneficiaries of the world’s largest vaccination drive.[1,2] In the early 2021, India approved two vaccines namely, Covaxin (manufactured by Bharat Biotech International Limited, Hyderabad, India) and Covishield (manufactured by Serum Institute of India Pvt. Ltd. Pune, India).[3]
With more and more vaccines getting approval, India now has five emergency approved vaccines with many other indigenous vaccines underway.[4,5] The vaccines approved in India are undergoing clinical trials and the data collected so far is promising with high efficacy and safety.[6] The most common side effects of the various vaccines were mild and short-lasting, with significant adverse effects being rare.[7]
The immunogenic effects of vaccines can lead to the development of various dermatoses and the covid-19 vaccines also seem to follow this dictum.[8] Cutaneous reactions were reported after messenger ribonucleic acid-based vaccines in the United States, with delayed local reactions being the most common.[9] Preliminary findings from trials of other vaccines from other parts of the world also report such manifestations.[10] There is a dearth of published literature describing cutaneous adverse effects of covid-19 vaccines in India with only two case series reporting a number of cutaneous reactions to Covishield and Covaxin.[11,12]
The lack of sufficient data on this topic has created apprehension and doubt among the general public as well as among HCWs. The present study aimed to fill this gap by describing the cutaneous manifestations developing after the two most commonly used covid-19 vaccines in India (Covishield and Covaxin) in HCW and the factors associated with the development of cutaneous manifestations. Furthermore, this study, explored the morbidity, if any, caused to the individuals with chronic dermatological disease due to vaccination.
MATERIALS AND METHODS
Study setting, period and design
A cross-sectional study was conducted over 3 months to find out any dermatological manifestations developed after receipt of covid-19 vaccine among HCWs, working at a tertiary care hospital in Delhi.
Sample size
No published literature for adverse dermatological manifestations post-covid-19 vaccination was available. Hence, assuming the prevalence of any adverse dermatological manifestation to be 50% (maximum possible for any disease), the minimum sample size came out to be 384 with an error rate of 5%.
Inclusion criteria
All vaccinated HCW working at the institution who gave consent were included in the study.
Data collection tool and method
Data collection was done with the help of a pretested, semi-structured and bilingual (English and Hindi) google form. The link for the google form was shared among the HCWs through various social media platforms. Those who could not be reached through these platforms were contacted for a telephonic or a physical interview.
The google form had information about the study and an e-consent was taken before starting the collection of information. Those who had participated in physical interviews were provided with all the information about the study and their consent was taken before data collection. Contact details of the primary investigator were provided to the participants who wanted any clarifications thereof. If someone preferred a telephonic interview over filling out a google form, they were contacted at their convenience and interviewed.
The first section of the questionnaire collected the demographic details of the participant. The next section asked about the vaccination details and history of covid-19 infection. The third section collected information about the dermatological manifestations (if any) experienced by the participant post-vaccination.
Ethical considerations
The study was initiated after receiving ethical clearance from the Institute Ethics Committee. Participants were enrolled in the study only after their informed consent had been obtained.
Data analysis
Data collected through google forms were summarised in an excel sheet. Data were analysed using SPSS, version 25 for windows available with the institution. Qualitative variables were expressed as proportions and quantitative variables were summarised as mean and standard deviation. Chi-square test, fisher’s exact test and t-test were used to determine if a relationship existed between the variables and P ≤ 0.05 was taken for declaring any significant difference.
RESULTS
Total 684 responses were received, among them 8 (1.16%) participants did not receive any covid-19 vaccine; hence, 676 participants were included in the final analysis. Out of 676, 429 (63.5%) received Covishield, 243 (35.9%) participants received Covaxin and 4 (0.6%) received Pfizer vaccine. Demographic characteristics and other parameters related to the study participants are presented in [Table 1].
| Characteristics | Number of participants (n) | Percentage |
|---|---|---|
| Sociodemographic characteristics | ||
| Gender | ||
| Male | 405 | 59.9 |
| Female | 271 | 40.2 |
| Mean age (years) | 22.9±6.9 | |
| Religion | ||
| Hindu | 603 | 89.2 |
| Muslim | 35 | 5.2 |
| Sikh | 11 | 1.6 |
| Christ | 15 | 2.2 |
| Buddhist | 12 | 1.8 |
| Designation | ||
| Undergraduate | 550 | 81.4 |
| Postgraduate | 64 | 9.5 |
| Consultants | 33 | 4.9 |
| Paramedical | 29 | 4.3 |
| Vaccination related characteristics | ||
| Vaccine doses received | ||
| Only first dose | 33 | 4.9 |
| Both first and second dose | 531 | 78.6 |
| First, second and booster | 112 | 16.6 |
| Type of vaccine | ||
| Covaxin | 243 | 35.9 |
| Covishield | 429 | 63.5 |
| Sputnik | 4 | 0.6 |
| Skin related previous disease status | ||
| Previous history of allergy to mask, gloves and sanitiser | ||
| No | 607 | 89.9 |
| Mask | 22 | 3.3 |
| Gloves | 16 | 2.4 |
| Sanitiser | 31 | 4.6 |
| Prior chronic dermatological condition | ||
| No | 626 | 92.6 |
| Acne/acne vulgaris | 14 | 2.1 |
| Psoriasis | 9 | 1.3 |
| Urticaria | 8 | 1.2 |
| Seborrhoeic dermatitis | 9 | 1.3 |
| Fungal infection | 3 | 0.4 |
| Vitiligo | 2 | 0.3 |
| Lichen planus | 2 | 0.3 |
| Medial canalicular dystrophy | 1 | 0.1 |
| Contact dermatitis | 1 | 0.1 |
| Sarcoidosis of skin | 1 | 0.1 |
| Autoimmune disease | ||
| No | 667 | 98.7 |
| Allergic rhinitis | 1 | 0.1 |
| Ankylosing spondylitis | 1 | 0.1 |
| Atopy | 1 | 0.1 |
| Dermatomyositis | 2 | 0.3 |
| Hashimoto thyroiditis and IBD | 2 | 0.3 |
| Liver disease | 1 | 0.1 |
| Sarcoidosis of lungs and skin | 1 | 0.1 |
IBD: Inflammatory bowel disease
Significantly more males and females were vaccinated with Covishield compared to Covaxin. (P = 0.030). Significant (P = 0.023) age difference was found among the recipients of Covishield and Covaxin with the mean age of getting vaccinated with Covishield being 23.26 ± 7.30 and for Covaxin being 22.3 ± 6.32 years. This is probably due to the fact that only Covaxin was approved for those below 18 years in the vaccination drive at the time of conducting this study. Among all groups of HCW studied, more subjects were vaccinated with Covishield than Covaxin. No statistically significant difference was found between the two vaccine groups in terms of sociodemographic and clinical parameters, as shown in [Table 2].
| Characteristic | Covaxin | Covishield | P-value |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Gender | |||
| Male | 159 (39.5) | 244 (60.5) | 0.030 |
| Female | 84 (31.2) | 185 (66.8) | |
| #Mean age (years) | 22.3±6.32 | 23.26±7.30 | 0.023 |
| *Religion | |||
| Hindu | 217 (36.1) | 384 (63.9) | 0.294 |
| Muslim | 15 (45.5) | 18 (54.5) | |
| Sikh | 01 (9.1) | 10 (90.9) | |
| Christ | 05 (33.3) | 10 (66.7) | |
| Buddhist | 05 (41.7) | 07 (58.3) | |
| Designation | |||
| Undergraduate | 212 (38.8) | 334 (61.2) | 0.006 |
| Postgraduate | 12 (18.8) | 52 (81.3) | |
| Consultants | 08 (24.2) | 25 (75.8) | |
| Paramedical | 11 (37.9) | 18 (62.1) | |
| Vaccine related variables | |||
| Vaccine doses received | |||
| Only first dose | 13 (40.6) | 19 (59.4) | 0.510 |
| Both first and second dose | 185 (35.0) | 343 (65.0) | |
| First, second and booster | 45 (40.2) | 67 (59.8) | |
| Other variables | |||
| History of allergy to PPE components | |||
| No allergy | 220 (36.5) | 383 (63.5) | 0.606 |
| Allergy to masks, gloves or sanitiser | 23 (33.3) | 46 (66.7) | |
| History of prior chronic dermatological condition | |||
| No | 229 (36.8) | 393 (63.2) | 0.212 |
| Yes | 14 (28.0) | 36 (72.0) | |
| *Autoimmune disease | |||
| No | 239 (36.0) | 424 (64.0) | 0.729 |
| Yes | 04 (44.4) | 05 (55.6) |
Out of 676 vaccine recipients, 14 (2.0%) subjects developed adverse dermatological manifestations including acne vulgaris, urticaria and increased hair loss. Types of different adverse dermatological manifestations developed post-vaccinations are given in [Figure 1]. Only 3.3% of the participants developed new skin manifestations after receiving Covaxin, while 1.4% developed new skin manifestations after receiving Covishield, (P = 0.224). Thus, no significant difference was observed between the developments of a new dermatological manifestation between the two vaccine groups. Development of adverse dermatological manifestation was found to be highest (10.3%) among paramedical staff (P = 0.018). Association between development of an adverse manifestation and other variables is given in [Table 3].
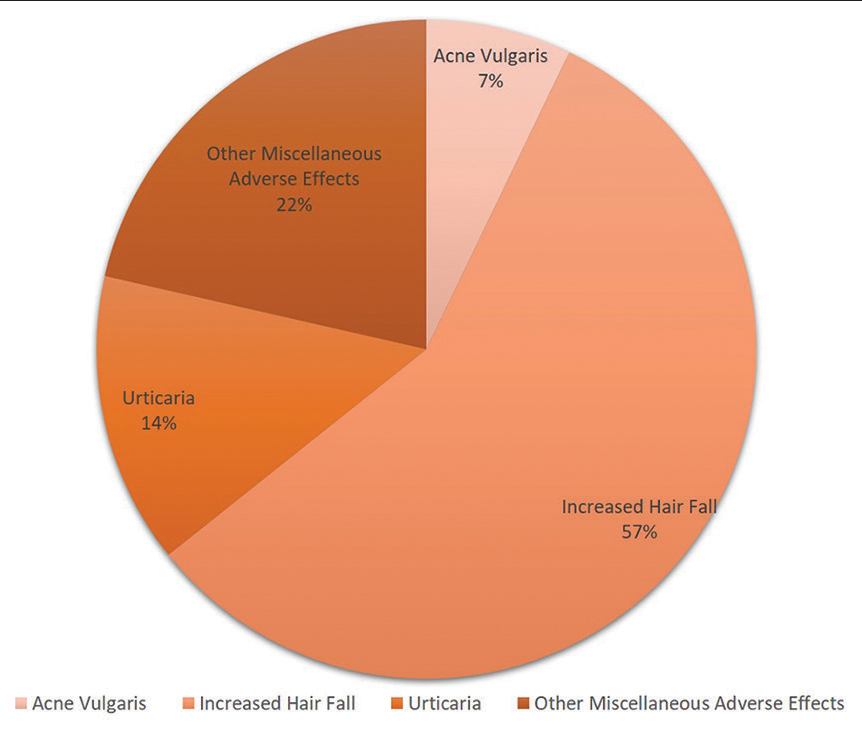
- New dermatological manifestations reported by the participants.
| Characteristic | No dermatological manifestation | New dermatological manifestation | P-value |
|---|---|---|---|
| Demographic profile | |||
| Gender | |||
| Male | 395 (97.5) | 10 (2.5) | 0.374 |
| Female | 267 (98.5) | 4 (1.5) | |
| #Mean age (years) | 22.73±6.9 | 25.43±9.4 | 0.153 |
| *Religion | |||
| Hindu | 590 (97.8) | 13 (2.2) | 0.801 |
| Muslim | 34 (97.1) | 01 (2.9) | |
| Sikh | 11 (100.0) | 0 (0.0) | |
| Christ | 15 (100.0) | 0 (0.0) | |
| Buddhist | 12 (100.0) | 0 (0.0) | |
| *Designation | |||
| Undergraduate | 542 (98.5) | 8 (1.5) | 0.018 |
| Postgraduate | 62 (96.9) | 2 (3.1) | |
| Consultants | 32 (97.0) | 1 (3.0) | |
| Paramedical | 26 (89.7) | 3 (10.3) | |
| *Type of vaccine received | |||
| Covaxin | 235 (96.7) | 8 (3.3) | 0.224 |
| Covishield | 424 (98.6) | 5 (1.4) | |
| Pfizer | 04 (100.0) | 0 (0.0) | |
| Vaccine related variables | |||
| *Vaccine doses received | |||
| Only first dose | 33 (100.0) | 0 (0.0) | 0.862 |
| Both first and second dose | 520 (97.9) | 11 (2.1) | |
| First, second and booster | 109 (97.3) | 3 (2.7) | |
| Other variables | |||
| *History of allergy to PPE components | |||
| No allergy | 596 (98.2) | 11 (1.8) | 0.164 |
| Allergy to masks, gloves or sanitiser | 66 (95.7) | 3 (4.3) | |
| *History of prior chronic dermatological condition | |||
| None | 612 (97.8) | 14 (2.2) | 0.615 |
| Yes | 50 (100.0) | 0 (0.0) | |
| *Autoimmune disease | |||
| None | 653 (97.9) | 14 (2.1) | 1.000 |
| Yes | 9 (100.0) | 0 (0.0) | |
Flare-up of previous disease conditions post-vaccination among participants is detailed in [Table 4]. Flare-up in symptoms was found in one patient with the previous contact allergic dermatitis to sanitiser, one case of acne and three cases of psoriasis. None of the systemic autoimmune diseases flared post-vaccination.
| Characteristics | n | Flare-up | |
|---|---|---|---|
| Yes | No | ||
| Previous history of allergy to mask, gloves and sanitiser (n=69) | |||
| Mask | 22 | 0 (0.0) | 22 (100.0) |
| Gloves | 16 | 0 (0.0) | 16 (100.0) |
| Sanitiser | 31 | 1 (3.2) | 30 (96.8) |
| Prior chronic dermatological condition (n=50) | |||
| Acne/acne vulgaris | 14 | 1 (7.1) | 13 (92.9) |
| Psoriasis | 9 | 3 (33.3) | 6 (66.7) |
| Urticaria | 8 | 0 (0.0) | 8 (100.0) |
| Seborrhoeic dermatitis | 9 | 0 (0.0) | 9 (100.0) |
| Fungal infection | 3 | 0 (0.0) | 3 (100.0) |
| Vitiligo | 2 | 0 (0.0) | 2 (100.0) |
| Lichen planus | 2 | 0 (0.0) | 2 (100.0) |
| Medial canalicular dystrophy | 1 | 0 (0.0) | 1 (100.0) |
| Contact dermatitis | 1 | 0 (0.0) | 1 (100.0) |
| Sarcoidosis of skin | 1 | 0 (0.0) | 1 (100.0) |
| Autoimmune disease (n=9) | None of the autoimmune disease showed flare-up in condition | ||
Figure in parenthesis indicate %
DISCUSSION
This study identified that only 2% of participants developed a dermatological adverse effect post-vaccination. A higher percentage of Covaxin recipients developed these adverse drug reactions as compared to the recipients of Covishield with no significant difference between the vaccine groups. Increased hair fall was the most common adverse effect reported.
Another study from northern India, by Das et al., reported a slightly lesser incidence (1.23%) of adverse cutaneous reactions to covid-19 vaccines. In their study, 50 patients reported an adverse cutaneous reaction to vaccines, the most common adverse event being telogen effluvium, followed by urticaria.[13] Bawane et al. reported a higher incidence of cutaneous reaction in Covishield recipients (86.7%) as compared to Covaxin (13.3%). This was in contrast to our study with a much lower overall incidence (2%) and no significant difference between the two vaccine groups.[14] A systematic review from Iran showed that reaction at the local injection site was the most common cutaneous adverse effect observed after Covid vaccination. Their study showed that most of the reactions were self-limiting and do not hamper vaccination.[15]
Limitations
Our study population included only HCW, out of which most (81.4%) were undergraduate students [Table 1]. Hence, the extrapolation of our results to the entire population is doubtful. Furthermore, the questionnaire method is dependent on the participant’s level of understanding and knowledge of the disease which can be a source of error in diagnosis of dermatological manifestations. Finally, while we tried to establish a temporal link between the vaccine and development of a new dermatological manifestation, further studies with more diverse study groups are needed to confirm this aspect. The lack of follow-up in our study also limits our understanding of the nature of the cutaneous reactions after vaccination.
CONCLUSION
This study identified an overall low incidence of dermatologic adverse effects following Covishield and Covaxin vaccines, with no significant difference in adverse events between the two vaccine groups. This study definitely adds to the existing knowledge regarding dermatological adverse effects post-covid-19 vaccines.
This study highlights the dermatological safety of the two vaccines.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- 30 Million Frontline Workers to Get covid-19 Vaccine in Phase 1 Hindustan Times. 2020. Available from: https://www.hindustantimes.com/india-news/30-million-frontline-workers-to-get-covid-19-vaccine-in-phase-1/story-w76e1pJ7toA4aod3hH234O.html [Last accessed on 2023 Jun 29]
- [Google Scholar]
- India Rolls out the World’s Largest covid-19 Vaccination Drive. 2021. Available from: https://www.who.int/india/news/feature-stories/detail/india-rolls-out-the-world-s-largest-covid-19-vaccination-drive [Last accessed on 2023 Jun 29]
- [Google Scholar]
- India’s Drugs Experts Approve AstraZeneca. 2021. Local Covid Vaccines. Available from: https://www.reuters.com/article/health-coronavirus-india-vaccine/indias-drugs-experts-approve-astrazeneca-local-covid-vaccines-idUSKBN29707B [Last accessed on 2023 Jun 29]
- [Google Scholar]
- Johnson and Johnson’s Jab Added to List: India Now Has 5 Vaccines for Adults. 2021. All You Need to Know:@cnnnews18. Available from: https://www.news18.com/news/india/with-moderna-added-to-list-how-many-covid-vaccines-does-india-have-till-now-all-you-need-to-know-3905555.html [Last accessed on 2023 Jun 29]
- [Google Scholar]
- Zydus Cadila: What We Know about India’s New covid Vaccines BBC News. 2021. Available from: https://bbc.com/news/world-asia-india-55748124 [Last accessed on 2023 Jun 29]
- [Google Scholar]
- Vaccine Information. 2021. ICMR New Delhi-covid-19 Vaccine. Available from: https://vaccine.icmr.org.in/covid-19-vaccine [Last accessed on 2023 Jun 29]
- [Google Scholar]
- Possible Side Effects After Getting a covid-19 Vaccine CDC: @CDCgov. 2021. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html [Last accessed on 2023 Jun 29]
- [Google Scholar]
- Cutaneous adverse reactions to covid-19 vaccines: Insights from an immuno-dermatological perspective. Vaccines (Basel). 2021;9:944.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous reactions reported after moderna and pfizer covid-19 vaccination: A registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- [CrossRef] [PubMed] [Google Scholar]
- Covid-19 Vaccines and the skin: The landscape of cutaneous vaccine reactions worldwide. Dermatol Clin. 2021;39:653-73.
- [CrossRef] [PubMed] [Google Scholar]
- Benign cutaneous reactions post-Covid-19 vaccination: A case series of 16 patients from a tertiary care center in India. J Cosmet Dermatol. 2022;21:30-3.
- [CrossRef] [PubMed] [Google Scholar]
- Exacerbation of pre-existing dermatoses following covid-19 vaccination: A case series from Eastern India. Indian J Postgrad Dermatol. 2023;1:47-50.
- [CrossRef] [Google Scholar]
- A study of Covid-19 vaccine (Covishield) induced dermatological adverse effects from India. J Eur Acad Dermatol Venereol. 2022;36:e402-4.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous adverse effects of the available Covid-19 vaccines in India: A questionnaire-based study. J Eur Acad Dermatol Venereol. 2022;36:e619-22.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous adverse reactions of Covid-19 vaccines: A systematic review. Dermatol Ther. 2022;35:e15391.
- [CrossRef] [PubMed] [Google Scholar]







