Translate this page into:
Resorcinol in Dermatology
*Corresponding author: Namrata Chhabra, Department of Dermatology, All India Institute of Medical Sciences (AIIMS), Raipur, Chhattisgarh, India. chhabra.namrata@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: George CA, Chhabra N. Resorcinol in Dermatology. Indian J Postgrad Dermatol. 2024;2:20-3. doi: 10.25259/IJPGD_108_2023
INTRODUCTION
Resorcinol has been used as an antiseptic and in low doses in keratolytic topical treatments in human medicine for over a century. It also exhibits antifungal and antipruritic properties.[1]
SOURCES
Plant phenolics, which include resorcinol ring-containing components, are abundant in nature and thoroughly studied. Broad bean, honey mushroom, yellow pond lily seedling exudates and tobacco leaf extracts have all been reported to have it. Resorcinol is a monomeric by-product of humic substance reduction, oxidation and microbial degradation.[2]
PHYSICAL AND CHEMICAL PROPERTIES
The International Union of Pure and Applied Chemistry (IUPAC) name of resorcinol is 1,3-dihydroxybenzene. Resorcinol is a phenol derivative in which a hydroxyl group substitutes a hydrogen atom in the meta position for the OH group[2] [Figure 1]. It has a bittersweet taste and is a white crystalline substance with a mild odour. In the presence of air and light, crystalline resorcinol becomes pale red, and it is hygroscopic and water soluble. Under ambient circumstances, the pKa values of 9.32 and 9.81 (at 25°C) show that resorcinol is exclusively present in the protonated form.[2]
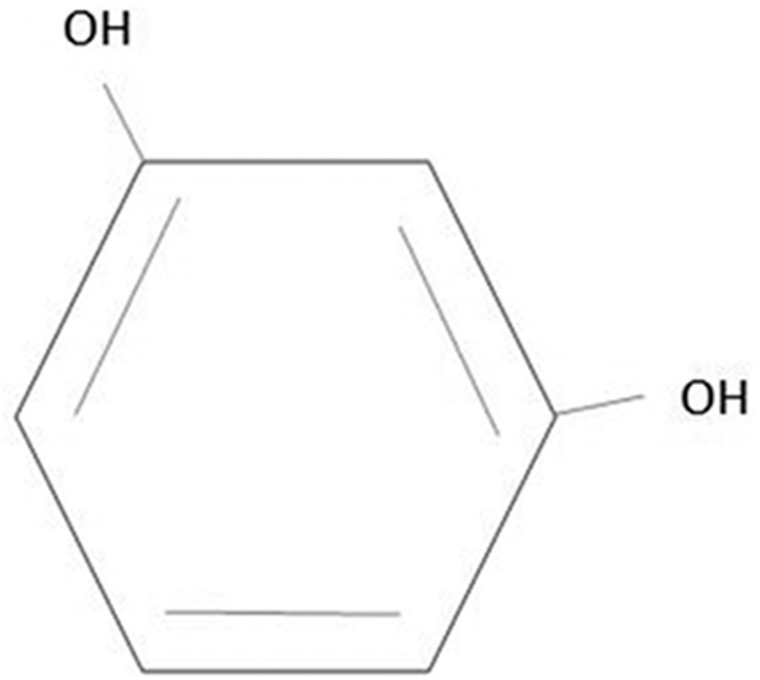
- Chemical structure of resorcinol.
PHARMACOKINETICS AND PHARMACODYNAMICS
Although there is no specific data on the half-life of resorcinol, an adult man with a 90% phenol exposure had an elimination half-life of roughly 14 h in cutaneous exposure. Resorcinol is absorbed by the oral, dermal and subcutaneous routes, is rapidly metabolised and is eliminated mostly as glucuronide conjugates in the urine. There is no evidence of bioaccumulation based on the research provided. The possibility for resorcinol absorption through undamaged skin using a hydroalcoholic carrier is limited.[2]
MECHANISM OF ACTION
Keratolytic action
The capacity of resorcinol to precipitate cutaneous proteins from the treated skin in different skin care topical treatments and peels appears to revolve around the compound’s capacity to cure acne, dermatitis and hidradenitis suppurativa (HS).[3] The follicular keratin plug, a probable initial event in all follicular occlusion disorders, is targeted by topical resorcinol. Keratin’s weak hydrogen bonds are disrupted by resorcinol and it has a significant concentration-dependent desquamation action. Practically, it is clear that resorcinol is a far more powerful comedolytic agent than existing keratolytic agents such as topical retinoids or azelaic acid.
Depigmenting action
The resorcinol derivative 4-n-butylresorcinol inhibits both tyrosinase and tyrosinase-related protein-1. The superiority of 4-n-butylresorcinol over other hypopigmented drugs was established by biochemical studies on the suppression of human tyrosinase activity.[4]
Anti-inflammatory and antipruritic action
It helps to reduce inflammation by promoting the formation of prostaglandin E.[5] Resorcinol peels permit non-traumatic drainage and lesion shrinkage.
Antimicrobial action
Resorcinol compounds exert fungicidal action by blocking fungal defence strategies, inducing the rupture or bursting of its defensive structures.[5]
USES IN DERMATOLOGY
In 1991, the Food and Drug Administration (FDA) published a final rule for over-the-counter topical acne medications that allowed the use of the other active ingredients listed in the 1985 list-resorcinol, resorcinol monoacetate, salicylic acid and/or sulphur in certain concentrations and combinations.
Resorcinol is the only peeling agent reported in the literature as a treatment for HS. It has been reported to produce an earlier breakthrough or resorption of the lesions in the acute phase of HS. The reduction of hyperkeratosis may improve drainage from the follicle during the maintenance phase when the lesions have settled down.[3] Topical 15% resorcinol has been increasingly reported to be safe and effective for mild-to-moderate HS. It could be a valid alternative to clindamycin in the management of acute and long-standing HS, limiting antibiotic use and, thereby, antimicrobial resistance.[6]
In one split-face study, 10% resorcinol in combination with 20% azelaic acid and 6% phytic acid was found to be as effective as 50% glycolic acid peel in the treatment of melasma.[7] Resorcinol has also been used in several other follicular occlusion disorders;[3,8] keratinisation disorders;[5] cutaneous infections[9] and eczema, pustular and papulosquamous disorders [Table 1].
| 1. | Follicular occlusion disorders Acne vulgaris Hidradenitis suppurativ Dissecting cellulitis of scalp |
| 2. | Disorders of hyperpigmentation Melasma Other hyperpigmentation disorders |
| 3. | Infections Fungal Tinea versicolor Dermatophytosis Fungal intertrigo Viral Viral warts |
| 4. | Pruritic disorders Pruritus ani Pruritus vulvae |
| 5. | Hyperkeratotic disorders Calluses Corns |
| 6. | Eczematous disorders Hyperkeratotic eczema Seborrhoeic dermatitis Stasis dermatitis |
| 7. | Palmoplantar pustulosis Pustular bacterids of hands and feet |
| 8. | Papulosquamous disorders Psoriasis |
DOSAGE AND FORMULATIONS
In doses ranging from 1% to 10%, it is available as an ointment, cream or lotion. It is also available in the form of compound resorcinol ointment, which contains 6% resorcinol. Resorcinol monoacetate slowly releases resorcinol, with a milder but long-lasting effect. Dosing of resorcinol is carefully titrated to reach a therapeutic concentration with minimum adverse effects. Initially, 10% cream is applied twice daily for the 1st week, then subsequently increased by 5% each week until lesions and pain begin to resolve, at which point the concentration is kept constant. The final dose is at least 15% cream as keratolysis is minimal below this concentration. Maintenance therapy involves prophylactic application twice weekly. In preparations for seborrhoea, resorcinol monoacetate is combined with sulphur at low dosages of 1–2%. Topical resorcinol as a peeling agent is available at concentrations of 5–15% in creams or drying pastes. Peeling agents or pastes for the treatment of leg ulcers have sometimes been used at significantly greater concentrations (up to 50%). The application of resorcinol to wounded skin is one element that increases the risk of toxic consequences.[10]
Resorcinol 2% in combination with sulphur (3–8%) and resorcinol monoacetate 3% in conjunction with sulphur (3–8%) are among the FDA-approved combinations.
Castellani’s paint[1] contains basic fuchsin 0.3%, ethyl alcohol (95%) 10%, boric acid 1%, phenol (liquefied) 4%, acetone 5% and resorcinol 10%.[1] Castellani’s paint is effective in inflamed tinea cruris and groin intertrigo, especially in individuals who have used topical steroids for a long time. Applying Castellani’s paint on oozing sores, especially in the groins and toe web spaces, is an effective way to dry them out.
Jessner’s peel (Combe’s formula)[10] is comprised of 14% each of resorcinol, salicylic acid and lactic acid mixed in ethanol. This combination enables more consistent penetration and peeling while using a lower and safer concentration.[10]
The formulations available in India are Jessner’s peel, resorcinol in combination with alpha arbutin, rumex occidentalis, liquorice extract as skin brightening gel, resorcinol 2% cream in combination with salicylic acid 3%, sulphur 2% and resorcinol 5% lotion in combination with sulphur 5% for acne.
ADVERSE EFFECTS
Adverse reactions to topical 15% resorcinol are mild, manifesting as mild irritation, desquamation and self-resolving brown hyperpigmentation [Figure 2]. Myxoedema and hypothyroidism have been reported particularly after repeated application on ulcerated regions. Although contact sensitisation appears to be uncommon, patch testing is required few days before the peel.[11] Because resorcinol has potent vasodilatory properties, patients who get resorcinol peels may experience lightheadedness and even syncope. Therefore, they should lie down throughout the peel and gently rinse once it is over.[10] Because of the potential for resorcinol toxicity, Jessner’s solution should be restricted to a small surface area (only face).
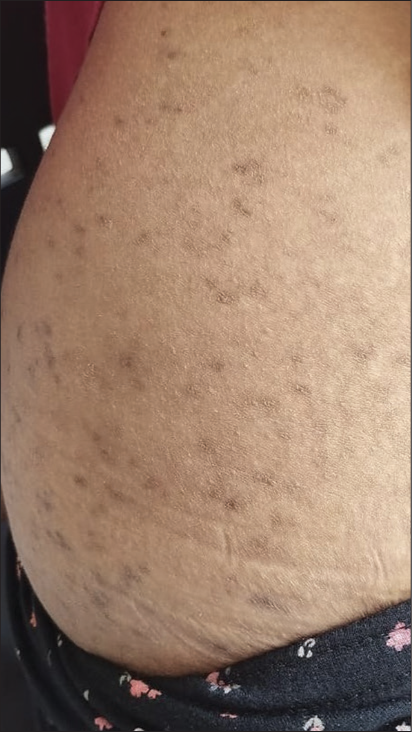
- Hyperpigmentation after application of resorcinol 5% combined with sulphur 5% lotion for truncal acne.
Red blood cells changes such as methemoglobinemia, haemolytic anaemia, hemoglobinuria and cyanosis have been documented in a few studies. To minimise significant absorption, resorcinol should not be applied to ulcerative and other open lesions, and special caution should be used in youngsters and patients with low body weight.[10]
Even in animal experiments, there was no evidence of carcinogenicity or mutagenicity. There has been no documented influence on reproductive performance or potential.[3]
CONTRAINDICATIONS
There are no listed absolute contraindications for dermatological use. There is no controlled data on pregnancy, paediatric or geriatric use. The FDA pregnancy category is not assigned to resorcinol.
CONCLUSION
Even though years old constituent of topical preparations, resorcinol continues to be in use due to its antiseptic, antimycotic, antipruritic and keratolytic properties in dermatology. Recent evidence in HS and acne vulgaris is of paramount significance due to the rising trend of antimicrobial resistance in these conditions.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Concise International Chemical Assessment Document 71 Vol 1. Geneva, Switzerland: World Health Organization; 2006. p. :3336-44.
- [Google Scholar]
- Dissecting Cellulitis of the Scalp Successfully Treated with Topical Resorcinol 15. Dermatol Ther. 2020;33:e13406.
- [CrossRef] [Google Scholar]
- Evidence-Based Treatment for Melasma: Expert Opinion and a Review. Dermatol Ther (Heidelb). 2014;4:165-86.
- [CrossRef] [PubMed] [Google Scholar]
- Antidermatophytic Action of Resorcinol Derivatives: Ultrastructural Evidence of the Activity of Phenylethyl Resorcinol against Microsporum gypseum. Molecules. 2016;21:1306.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and Safety of Topical Resorcinol 15% Versus Topical Clindamycin 1% in the Management of Mild-to-moderate Hidradenitis Suppurativa: A Retrospective Study. Dermatol Ther. 2022;35:e15439.
- [CrossRef] [Google Scholar]
- Solution of Azelaic Acid (20%), Resorcinol (10%) and Phytic Acid (6%) Versus Glycolic Acid (50%) Peeling Agent in the Treatment of Female Patients with Facial Melasma. Adv Biomed Res. 2017;6:9.
- [CrossRef] [PubMed] [Google Scholar]
- Over-The-Counter Treatments for Acne and Rosacea. Semin Cutan Med Surg. 2016;35:87-95.
- [CrossRef] [PubMed] [Google Scholar]
- Cumulated Index Medicus U.S. United States: Department of Health and Human Services, Public Health Service, National Institutes of Health, National Library of Medicine. 1964. p. :1388.
- [Google Scholar]
- Peeling Agents: Toxicological and Allergological Aspects. J Eur Acad Dermatol Venereol. 1999;13:14-23.
- [CrossRef] [PubMed] [Google Scholar]
- Manual of Chemical Peels: Superficial and Medium Depth Philadelphia, PA: Lippincott Williams and Wilkins; 1995. p. :200.
- [Google Scholar]







