Translate this page into:
Imiquimod: Newer Perspectives to an Old Drug
*Corresponding author: Sonali Gupta, Department of Dermatology, Smt. Nathiba Hargovandas Lakhmichand (NHL) Medical College, Ahmedabad, Gujarat, India. dr.sonali42@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gupta S, Agarwal P, Baxi K, Nyati A. Imiquimod: Newer Perspectives to an Old Drug. Indian J Postgrad Dermatol. 2024;2:10-6. doi: 10.25259/IJPGD_99_2023
Abstract
Imiquimod is a topical age-old drug belonging to the imidazoquinolones class which acts by modulating the immune system. It has been approved by the US Food and Drug Administration for treating external genital and perianal warts, actinic keratoses and superficial basal cell carcinoma. Along with these, there have been a large number of diseases for which the drug has played a beneficial role with a good safety profile.
Keywords
Actinic keratosis
Anogenital warts
Imiquimod
Superficial basal cell carcinoma
INTRODUCTION
Imiquimod is a synthetic compound that belongs to the imidazoquinolones class of drugs. It acts as an immune response modifier in the body and also has potent anti-viral and anti-tumour activity. It’s role as an immune response modifier was discovered while screening various drugs for anti-herpetic activity.[1]
PHARMACOKINETICS
Topical imiquimod has minimal systemic absorption. Less than 0.9% of the dose is excreted in the urine and faeces.[1] The systemic exposure of drug is more dependent on the surface area of application than the amount of applied dose. The apparent half-life of the 5% cream applied topically is about 10 times greater as compared to the half life seen after following subcutaneous injection, which suggests its prolonged retention after topical application.
CONCENTRATIONS AVAILABLE AND ADMINISTRATION
Imiquimod is available in 2.5%, 3.75% and 5% concentration.
A thin layer of cream is to be applied and left overnight for 6–10 hours (Centres for Disease Control and Prevention guidelines), after which the area is washed with mild soap and water to remove any cream.
Anogenital warts – Application is for 3 days a week overnight up to a maximum of 16 weeks
Basal cell carcinoma (BCC) – Once a day for 5 days a week on the lesion and the immediate surrounding area for 6 weeks.
Actinic keratosis (AK) – Should be applied for 2 days a week for 16 weeks. It should not be applied to an area larger than patient’s forehead or cheek (about 2 inches by 2 inches).
MECHANISM OF ACTION
Two mechanisms have been attributed to the efficacy of imiquimod in dermatology. They include immunomodulatory and anti-tumour (direct/indirect) actions.
Immunomodulatory action
Imiquimod acts as an agonist on toll-like receptors 7 and 8, which through a cascade of events leads to the induction of proinflammatory cytokines and chemokines along with activation of antigen presenting cells and innate immunity [Figure 1] causes activation of nuclear factor-kappa B which leads to the induction of proinflammatory cytokines and chemokines such as interferon-gamma (IFN-g), interferon-alpha and interleukin-12 (IL-12). This further leads to the activation of antigen-presenting cells and other components of innate immunity and, eventually, a profound T-helper (Th1) cellular immune response gets initiated. The mounting of T-helper Type 2 (Th-2) immune response is simultaneously inhibited.

- Immunomodulatory mechanism of imiquimod.
It also induces the migration of Langerhans cells to regional lymph nodes and enhances the antigen presentation to naive T-cells and thus perpetuating the cycle of T-cell response.
Anti-tumour action
Direct action
This is the main anti-tumour mechanism of imiquimod. Apoptosis in tumour cell lines is induced by activation of the workhorses of apoptosis (the caspase family of proteases) by intrinsic pathway.[2] It can also independently upregulate proapoptotic proteins of the Bcl-2 family of proteins [Figure 2].
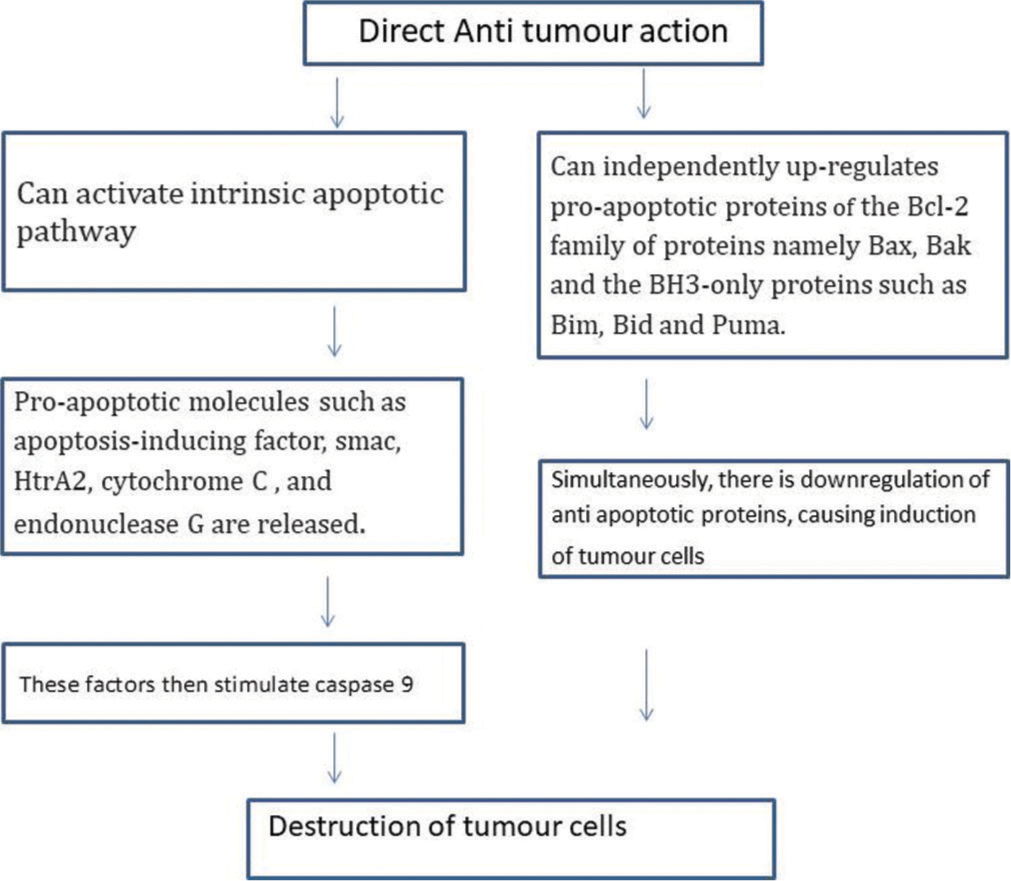
- Direct anti-tumour action mechanism of imiquimod.
Indirect actions
Indirect actions of imiquimod occur by inducing the release of various cytokines which, then, stimulate a cell-mediated immune response and contribute in drug’s anti-tumour activity.[3] The main cytokines induced include IL-12, tumour necrosis factor-alpha and IFN-g. These cytokines block angiogenesis and increase the levels of cytotoxic T-cells and natural killer cells in the local milieu by induction of 2’-5’-oligoadenylate synthetase.[4] Further, there has been an increase in IL-12 which down-regulates IL-10 and thus stimulates anti-tumour T-cells which are responsible for killing malignant cells.
INDICATIONS
Details of each have been described below [Table 1].
| FDA approved | Off label dermatological indications |
| Anogenital warts | Common warts |
| Actinic keratoses | Molluscum contagiosum |
| Superficial basal cell carcinoma | Keloids |
| Nodular and morphoeic basal cell carcinoma | |
| Squamous cell carcinoma in situ | |
| Invasive squamous cell carcinoma | |
| Melanoma | |
| Mycosis fungoides | |
| Infantile haemangioma |
FDA: Food and drug administration
Anogenital warts
The first clinical application in which imiquimod proved useful was for genital warts. The common therapeutic problems with anogenital warts are recurrence and long duration. The reason for this could be that most treatment therapies target physical destruction of the disease rather than direct antiviral activity against the human papillomavirus. Imiquimod represents a significant advancement in the treatment due to its antiviral and non-destructive properties. It has been Food and Drug Administration (FDA) approved in 5% cream formulation for overnight application (6–10 h) over warts 3 times a week for up to 16 weeks and 3.75% daily for up to 8 weeks (~50% clearance and ~30% clearance, respectively).[5] Although there have been various modalities of treatment such as excision, electrocautery and laser vaporisation which can be used to remove warts quickly, there have been many disadvantages as they are painful, destructive and recurrences are common with these modalities (i.e., occurring in 9–72% of warts treated with ablative laser therapy).[6] Imiquimod 5% cream showed some clearance of warts in immunosuppressed human immunodeficiency virus-infected patients with genital warts also. Major advantage of imiquimod lies in its self-application at home which decreases the hospital visits and increases compliance of the patient toward treatment. By virtue of increase in cellular immunity, it gives long-term protection against recurrences or reinfection[1] and, hence, serves as a better alternative for genital warts [Table 2].[7-15]
| Topical medication | Imiquimod cream | Podophyllotoxin | Podophyllin | Cryotherapy | Tri chloroacetic acid |
|---|---|---|---|---|---|
| Duration of use | 4-16 weeks | Upto 4 weeks | Upto 6 weeks | Upto 7 weeks | Upto 7 weeks |
| Mode of administration | Self administered | Self administered | Applied by a healthcare professional | Applied by a healthcare professional | Applied by a healthcare professional |
| Course of therapy | 3 times a week before bedtime, duration depends on response and wart clearance. | Apply twice daily for 3 consecutive days, followed by 4 days of no therapy | Apply every 1 to 2 weeks with vaseline in the periphery and left on for 4–6 h before washing off | Freezing of each wart is done separately and repeated every week | Multiple applications are done over a week interval |
| Clearance rate | Approx 56%[7] | 45 to 77%[8] | 30-80%[12] | 79-88% | 70-80%[10] |
| Recurrence rate | 13%[7] | 38%[9] | Variable | 25-40%[15] | 36%[11] |
| Side effects | Local skin reactions (redness, itching, burning) - Flu-like symptoms (rarely) | Skin irritation, redness, or burning -Mild flu-like symptoms (rarely) | Skin irritation, ulceration, or burning -Potential systemic toxicity (rarely)[13,14] | Pain or discomfort during the procedure - Blistering or ulceration of the treated area (temporary) | Skin irritation, redness, or burning -Potential for scarring |
Actinic keratoses
Imiquimod 5% cream was first approved by the FDA in 2004 and all the three concentrations have been approved. Application of 3.75% cream daily for 2 weeks have shown similar efficacy with fewer cutaneous adverse effects as 5% cream twice weekly for up to 16 weeks (~50% and ~35% clearance, respectively). Another added advantage witnessed with the lower concentration of imiquimod was the wider surface area of application (i.e., 200 cm2 as compared to 25 cm2 for the 5% preparation of imiquimod).[16]
Imiquimod produces its activity through augmented antigen recognition and amplified cellular immunologic response which causes development of immunologic memory and serves to reduce the development of recurrent or new AK. Field cancerization theory postulates that a large area of skin surrounding AK is also at increased risk for possible malignant transformation because it has been exposed to the same ultraviolet light for a long time. Thus, those therapies which work in a larger field have the potential to address subclinical damage, reducing AK recurrence rates and potentially reducing the risk of squamous cell carcinoma development.
There are various treatment regimens available in AK
Conventional – Imiquimod 5% cream to be applied thrice/week (every alternate day) for about 8–16 weeks.[4]
Modified regimen – Imiquimod 5% cream to be applied thrice/week every alternate day for 4 weeks which is followed by rest for 4 weeks. This whole process constitutes 1 cycle. Up to three cycles have shown better results with complete remission and lesser cutaneous side effects than the conventional regimen.[17]
Combination with cryotherapy – One session of cryotherapy consists of two sprays of liquid nitrogen for 5 second each with a rest period of 5 s in between sprays. This is followed by 3.75% imiquimod cream daily for 2 weeks which is followed by 2 weeks drug-free period after which cream is again applied daily for 2 weeks.[18]
The comparison of different modalities for AK is shown in Table 3.
| Topical medication | Dosage form | Treatment duration | Application frequency | Common side effects | Clearance rates | Recurrence rates |
|---|---|---|---|---|---|---|
| 5- Fluorouracil | Cream | 2 to 4 weeks | Once or twice daily, as directed | Local skin reactions (redness, peeling) |
67-96% | 54% |
| Imiquimod | Cream | 6 to 16 weeks | 3 times a week | Local skin reactions (redness, itching) |
Approx. 68% | 10.5% |
| Ingenol mebutate | Gel | 2 or 3 consecutive days | Single course application | Local skin reactions (redness, swelling) | Approx. 48% | - |
| Diclofenac | Gel | 60 to 90 days | Twice daily, as directed | Local skin reactions (redness, itching) |
Approx. 75% | - |
Superficial BCC
BCC is a common cutaneous neoplasm that very rarely metastasises, but if neglected, it can have a locally aggressive course with destruction of underlying tissues. Although Mohs micrographic surgery remains the gold standard for the treatment of BCC, imiquimod can also be used in selected cases. For BCC, an additional mechanism of imiquimod has been suggested which occurs by blocking the activation of the Hedgehog/glioma-associated oncogene (GLI) signalling pathway (important in the pathogenesis of BCC).[19]
Dosing – 5% imiquimod cream applied 5 times a week for 6 weeks (~75% clearance) in immunocompetent adults have been approved by FDA for superficial BCC with maximum tumour diameter <2 cm in 2004.[20] Thicker lesions have a low response rate. Imiquimod provides a good alternative for the people who are unwilling or unfit for surgery, although the success rate is lower than Mohs micrographic surgery (99%). When compared to photodynamic therapy, imiquimod may be more effective in treating superficial BCC [Table 4]. Imiquimod has shown excellent results in patients with multiple BCC and in xeroderma pigmentosum patients with only minor side-effects.
| Topical treatment | Dosage form | Treatment duration | Application frequency | Common side effects | Clearance rates | Recurrence rates |
|---|---|---|---|---|---|---|
| Imiquimod 5% | Cream | 6 to 12 weeks | 5 times a week | Local skin reactions (redness, itching) | Approx. 86% | 2% |
| 5-Fluorouracil | Cream | 2 to 6 weeks | Twice daily | Local skin reactions (redness, peeling) | Approx. 93% | 18% |
| Diclofenac | Gel | 6 to 12 weeks | Twice daily, as directed | Local skin reactions (redness, itching) | 64% | - |
| Photodynamic therapy | Solution | 1 to 2 sessions | Single application or with light therapy | Local skin reactions (burning, stinging) | 87% | 14% |
OFF LABEL DERMATOLOGICAL USES
Common warts
Imiquimod monotherapy has proved to be less efficient for treating cutaneous warts due to its decreased penetrance and absorption through wart tissue. Hence, combining it with other modalities of cytodestructive treatments such as cryotherapy, salicylic acid and occlusion can increase its penetrance thus increasing its efficiency and hence, better outcome of common warts. It may also serve as a good alternative in the management of recalcitrant warts in immunosuppressed patients.
Molluscum contagiosum
Molluscum is generally regarded as a self-limiting disease; however, its treatment is generally advised as it has a potentially protracted course and due to the risk of superinfection, scarring, autoinoculation and transmission to other members of the community. Topical imiquimod once daily under occlusion has shown remission within 3–8 weeks of treatment with only mild-to-moderate irritation in the application area.[21]
Keloids
Imiquimod causes an indirect increase in the production of interferon alpha and, possibly, transforming growth factor beta-3 at the site of application which decreases collagen and extracellular matrix component deposits involved in scar formation. It acts as a useful adjunct to surgical resection by decreasing recurrence. It is applied once daily for around 8 weeks at the site of surgical excision of the keloid has shown minimal recurrences following excision.[22]
Nodular and morphoeic basal cell carcinoma
Imiquimod, 5% cream can be used as an alternative in patients with small nodular and morphoeic BCCs who are poor candidates for surgery. According to a study, topical 5% imiquimod cream is well tolerated and most effective in treating nodular BCC when applied once daily for 6–12 weeks.[23]
Cutaneous malignancies other than BCC
Imiquimod has been found to be useful in many cutaneous malignancies and can be used as a standalone or as an adjunct to on-going treatment [Table 5].[24-26]
| Type of lesion | Treatment protocol | Treatment duration | Response |
|---|---|---|---|
| Bowen disease of skin | Imiquimod 5% cream once daily application | upto 16 weeks | Most patients had resolved and reported no residual SCC in situ in the post treatment biopsy specimen 6 weeks after stopping treatment |
| Vulvar intraepithelial neoplasia | Imiquimod was applied to lesion *3-5 days/wk along with application over 1cm of normal skin |
upto 16 weeks | Most of the patients showed complete response while some had moderate response. Though surgery is currently the standard of care for the treatment of VIN but for those patients who want to preserve sexual function, conservative treatment like imiquimod is beneficial.[24] |
| Erythroplasia of Queyrat (EQ) |
Imiquimod 5% to be applied 3-7 times/week |
upto 24 weeks | Good clinical response noted |
| Anal intraepithelial neoplasia | Imiquimod 5% to be applied 3 times per week | 16 weeks | Good initial response rates and there was improvement in HPV type and DNA load. Combination of topical and invasive procedures could offer the best benefit for treatment of AIN.[25] |
| Invasive SCC | Imiquimod 3 times a week | 12 weeks | Good response with no evidence of residual tumour at 6 months follow up. |
| Metastatic melanoma | 3 times weekly imiquimod as an adjunct with continuing treatment. 3 times a week application as a stand alone treatment | 12 weeks 16 weeks |
Imiquimod caused complete clearing in imiquimod- treated cutaneous metastatic lesions. Complete clearance of the lesions with recurrence at 15 months follow up.[26] |
VIN: Verrucous intraepithelial neoplasia, HPV: Human papilloma virus, AIN: Anal intraepithelial neoplasia, SCC: Squamous cell carcinoma
Mycosis fungoides
Imiquimod has proved to be an effective therapy for early-stage disease of cutaneous T-cell lymphomas, whether used alone or in combination with systemic immunomodulatory therapy. This is well tolerated and associated with a histologic and clinical response rate of 50% in patch and plaque stage.[27]
Infantile haemangioma (IH)
It has been reported to be efficacious in the treatment of IH due to its ability to induce the production of tissue inhibitor of matrix metalloproteinase which has antiangiogenesis property. There has been significant improvement in superficial IH and little improvement in mixed IH with no or very less changes in deep hemangiomas.[28]
CONTRAINDICATIONS
There are no known absolute contraindications to the use of imiquimod.
Relative contraindications include:
Patients with known benzyl alcohol hypersensitivity or paraben hypersensitivity
Pregnant and lactating females
Patients with pre-existing autoimmune diseases
Photosensitivity
Graft versus host disease.
ADVERSE EFFECTS
There have been no serious side effects of imiquimod application. Most frequently reported side effects include transient erythema, pruritus and burning sensation over the site of application. Dizziness, fatigue and angioedema have been reported by very few people [Table 6].
| Most frequent | Less frequent | Rare |
|---|---|---|
| Erythema | Diarrhoea | Dizziness |
| Pruritus | Headache | Fatigue |
| Burning sensation | Myalgia | Fever |
| Scaling and dryness | Upper respiratory tract infection |
Lymphadenopathy |
| Flu like symptoms | Severe erythema | Angioedema |
PRECAUTIONS
Treated area should not be covered with tight bandage or dressing
Sexual (oral, anal and genital) contact should be avoided while the cream is on the skin as imiquimod cream may weaken condoms and vaginal diaphragms
Uncircumcised men should pull the foreskin back and clean it daily and before each treatment
It should not be applied in or near the eyes, lips, nostrils, vagina or anus
Patients should be asked to dispose of any open packets
In case of severe local skin reaction to cream, the cream should be removed and area is to be washed with mild soap and water and resumed after the skin reaction has subsided.
CONCLUSION
Imiquimod being topical, is easy to use, self administered thus decreases the number of hospital visits and increases the compliance. It has good tolerability profile with the option of breaks from treatment as required for local skin reactions which makes it a good non invasive alternative as compared to the other destructive modalities available all together. Also in patients, who have failed to respond to other therapeutic modalities or who have had recurrence following standard therapy, or who are medically unfit for surgery, imiquimod may be beneficial with a close follow up routine.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Imiquimod: A Potential Role in Dermatology? Br J Dermatol. 2002;147:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Imiquimod: Unexpected Killer. J Invest Dermatol. 2004;122:15-6.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic Response of Basal Cell Carcinoma to the Immune Response Modifier Imiquimod 5% Cream. J Am Acad Dermatol. 1999;41:1002-7.
- [CrossRef] [PubMed] [Google Scholar]
- Imiquimod 5 Percent Cream and the Treatment of Cutaneous Malignancy. Dermatol Online J. 2004;10:4.
- [CrossRef] [PubMed] [Google Scholar]
- Safety, Efficacy, and Recurrence Rates of Imiquimod Cream 5% for Treatment of Anogenital Warts. Skin Ther Lett. 2009;14:1-3, 5
- [Google Scholar]
- Human Papillomavirus Infection and Genital Warts: Update on Epidemiology and Treatment. Clin Infect Dis. 1995;20(Suppl 1):S91-7.
- [CrossRef] [PubMed] [Google Scholar]
- Self-Administered Topical 5% Imiquimod Cream for External Anogenital Warts. HPV Study Group. Human Papillomavirus. Arch Dermatol. 1998;134:25-30.
- [CrossRef] [PubMed] [Google Scholar]
- Randomised Controlled Trial and Economic Evaluation of Podophyllotoxin Solution, Podophyllotoxin Cream, and Podophyllin in the Treatment of Genital Warts. Sex Transm Infect. 2003;79:270-5.
- [CrossRef] [PubMed] [Google Scholar]
- Podophyllotoxin 0.5% v Podophyllin 20% to Treat Penile Warts. Genitourin Med. 1988;64:263-5.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of External Genital Warts Comparing Cryotherapy (Liquid Nitrogen) and Trichloroacetic Acid. Sex Transm Dis. 1993;20:344-5.
- [CrossRef] [PubMed] [Google Scholar]
- Cryotherapy Compared with Trichloroacetic Acid in Treating Genital Warts. Genitourin Med. 1987;63:390-2.
- [CrossRef] [PubMed] [Google Scholar]
- Quercetin and Kaempherol: An Argument against the Use of Podophyllin? Genitourin Med. 1995;71:92-3.
- [CrossRef] [PubMed] [Google Scholar]
- An Evidence-based Review of Medical and Surgical Treatments of Genital Warts. Dermatol Online J. 2006;12:5.
- [CrossRef] [Google Scholar]
- Cycle Therapy of Actinic Keratoses of the Face and Scalp with 5% Topical Imiquimod Cream: An Open-label Trial. J Am Acad Dermatol. 2002;47:571-7.
- [CrossRef] [PubMed] [Google Scholar]
- An Investigator-Initiated Study to Assess the Safety and Efficacy of Imiquimod 3.75% Cream When Used after Cryotherapy in the Treatment of Hypertrophic Actinic Keratoses on Dorsal Hands and Forearms. J Clin Aesthet Dermatol. 2013;6:36-43.
- [CrossRef] [Google Scholar]
- Imiquimod Directly Inhibits Hedgehog Signalling by Stimulating Adenosine Receptor/Protein Kinase A-Mediated GLI Phosphorylation. Oncogene. 2013;32:5574-81.
- [CrossRef] [PubMed] [Google Scholar]
- Topical Imiquimod is an Effective and Safe Drug for Molluscum Contagiosum in Children. Acta Dermatovenerol Croat. 2017;25:164-6.
- [Google Scholar]
- Efficacy of Topical 5% Imiquimod Cream for the Treatment of Nodular Basal Cell Carcinoma Comparison of Dosing Regimens. Arch Dermatol. 2002;138:1165-71.
- [CrossRef] [PubMed] [Google Scholar]
- Imiquimod 5% Cream Versus Cold Knife Excision for Treatment of VIN 2/3: A Five-year Follow-up. Eur Rev Med Pharmacol Sci. 2013;17:936-40.
- [Google Scholar]
- A Double-blind, Randomized Controlled Trial of the Use of Imiquimod Cream for the Treatment of Anal Canal High-grade Anal Intraepithelial Neoplasia in HIV-Positive MSM on HAART, with Long-term Follow-up Data Including the Use of Open-label Imiquimod. AIDS. 2010;24:2331-5.
- [CrossRef] [PubMed] [Google Scholar]
- Topical Imiquimod in the Treatment of Metastatic Melanoma to Skin. Arch Dermatol. 2003;139:273-6.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of Patch and Plaque Stage Mycosis Fungoides with Imiquimod 5% Cream. J Am Acad Dermatol. 2005;52:275-80.
- [CrossRef] [PubMed] [Google Scholar]
- Topical Imiquimod in the Treatment of Infantile Hemangiomas: A Retrospective Study. J Am Acad Dermatol. 2007;56:63-8.
- [CrossRef] [PubMed] [Google Scholar]







