Translate this page into:
Cutaneous Alternariosis in a Patient with Pulmonary Granulomatosis with Polyangiitis (GPA): Is it a Marker of Immunosuppression or Immune Dysregulation?
*Corresponding author: Shital Patil, Department of Pulmonary Medicine, Venkatesh Chest Hospital, Latur, Maharashtra, India. drsvpatil1980@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Malhotra P, Unni MM, Patil S. Cutaneous Alternariosis in a Patient with Pulmonary Granulomatosis with Polyangiitis (GPA): Is it a Marker of Immunosuppression or Immune Dysregulation? Indian J Postgrad Dermatol. 2025;3:46-8. doi: 10.25259/IJPGD_161_2024
Abstract
Granulomatosis with polyangiitis (GPA) is a rare multisystem disorder of autoimmune pathology involving small- and medium-vessel vasculitis of respiratory and renal system. Immune dysregulation due to GPA illness and immunosuppression after use of immunomodulators predisposes to opportunistic secondary infections. We report a 43-year-old male who presented with acute respiratory distress and acute hypoxic respiratory failure, skin lesions and multifocal nodules with radiological bronchus sign on high-resolution computed tomography thorax imaging and negative workup for sepsis screen on microbiological workup. He was diagnosed as GPA after positive anti C-antineutrophil cytoplasmic antibody test. Skin lesions needle aspirate for potassium hydroxide mount showed fungal aetiology. Punch biopsy for histopathology and culture with fungal polymerase chain reaction analysis finally confirmed the diagnosis of cutaneous alternariosis. Combination regimen of methylprednisolone, azathioprine and itraconazole was used for the management of GPA with cutaneous alternariosis. We observed remission of GPA and documented complete resolution of lung and skin lesions. We recommend a high index of suspicion during management of GPA with concurrent fungal infection for successful treatment outcome.
Keywords
Cutaneous alternariosis
high-resolution computed tomography thorax
Polymerase chain reaction
Pulmonary granulomatosis with polyangiitis
Potassium hydroxide mount
INTRODUCTION
Granulomatosis with polyangiitis (GPA) is uncommon autoimmune multisystem disorder of unknown aetiology typically involving small and medium sized vessels vasculitis characterised by non-caseating granulomas. Previous nomenclature of ‘Wegener’s granulomatosis’ disease was changed in 2011 and is now called as GPA.[1] Although a significant proprotein of GPA cases present as localised disease, majority of them translate into generalised disease throughout illness and two-third of them manifest lung involvement as a natural course of disease.
CASE REPORT
A 43-year-old male, farmer, tobacco addict, without any known comorbidity admitted in intensive care unit with acute respiratory distress and skin lesion in intensive care unit. He was never exposed to immunosuppressive drugs before this presentation or having any comorbidity resulting in to it. General vital parameters were unstable with oxygen saturation which was 82% on room air and 94% on oxygen supplementation @ 3 L/min and blood pressure was 100/60 mmhg. Respiratory system examination revealed adventitious sounds as bilateral crepitations. High resolution computed tomography (HRCT) thorax showed bilateral, multiple well defined round consolidations with air bronchogram and masses in middle and lower lobes [Figure 1]. Skin lesions were multiple, ill-defined erythematous plaques approximately 1–2 cm in size with collarette scales all over the chest and back with few pustules over right chest and right flanks [Figure 2a], and over bilateral legs and anterior aspect of the forearm approximately 1–2 cm in size with scab formation [Figure 2b]. Laboratory examinations showed leucocytosis, normal blood sugar level, CD4 count is 460/uL, thyroid, liver and kidney functions were normal. myleoperoxidase (MPO)-Antineutrophil cytoplasmic antibody (ANCA) (P-ANCA) was 3.56 RU/mL (normal range 0–20 RU/mL) and proteinase3 (PR3)-Antineutrophil cytoplasmic antibody (ANCA) (C-ANCA) was >300 RU/mL (normal range 0–20 RU/mL). Sputum examination for acid fast bacilli was negative. Purulent, yellowish green secretions aspirated from nodule over anterior aspect of knee, anterior aspect of tibia and forearm in potassium hydroxide (KOH) mount examination noted fungal element with hyaline septate hyphae, and Gram stain and Ziehl-Nelson stain were inconclusive for microbiological workup. Histopathological examination of skin lesions revealed an intense inflammatory process in the dermis, and several septate hyphae and round or oval spore-like structures that stained positively with periodic acid Schiff stain [Figure 3]. Fungal culture in special chloramphenicol Sabouraud agar medium grown Alternaria species Alternaria alternata which was confirmed by performing polymerase chain reaction (PCR) using primers for deoxyribonucleic acid sequences of nuclear ribosomal internal transcribed spacer-1 region.
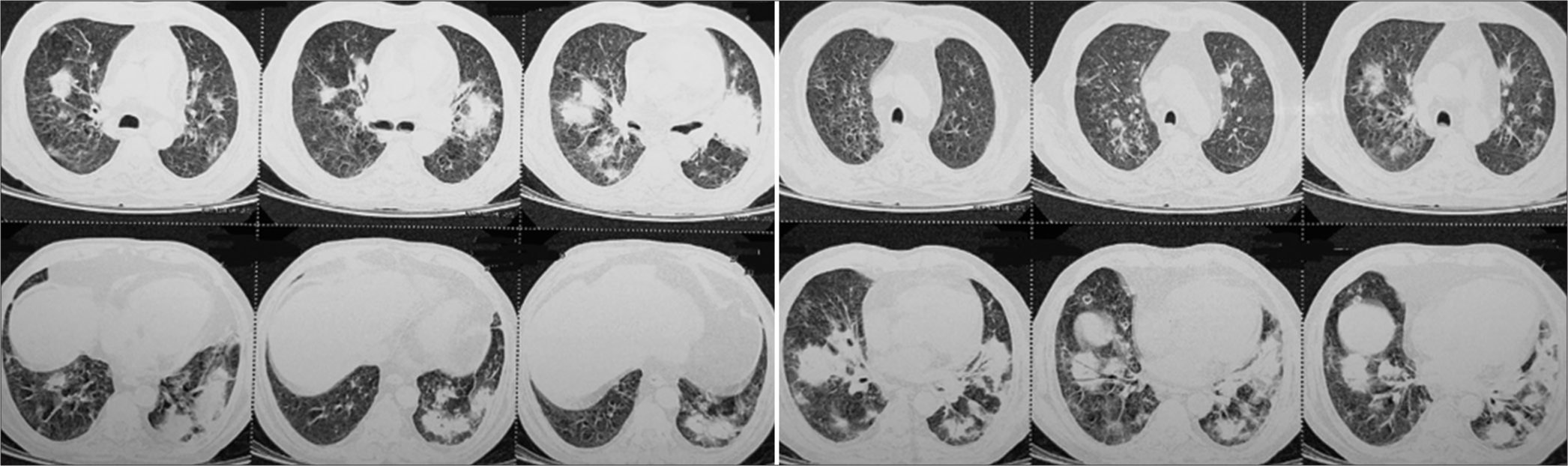
- High-resolution computed tomography thorax showing bilateral, multifocal, well-defined round consolidations in middle and lower lobes with air-bronchogram or positive bronchus sign.
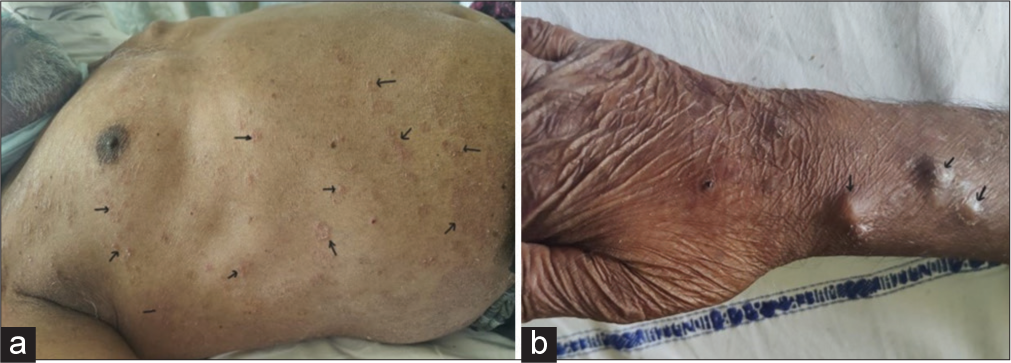
- (a) Showing ill-defined erythematous plaques over anterior and posterior aspect of trunk and chest wall (black arrows), (b) showing multiple well-defined skin-coloured nodules noted over anterior aspect of forearm and wrist (black arrows).
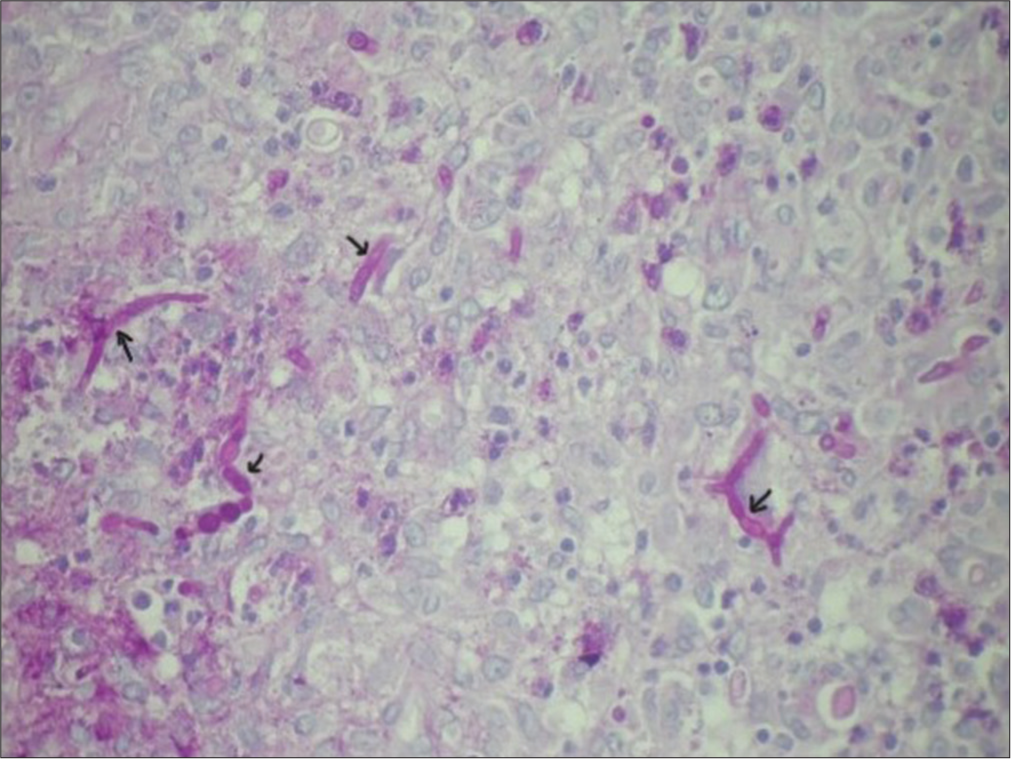
- Histopathology showing segmented hyphae and spherical spore-like bodies in the dermis (black arrows). Periodic acid-Schiff stain ×40.
Combinations regimen of methylprednisolone (MPS), Azathioprine (AZT) and Itraconazole was used for management of GPA with Cutaneous Alternariosis and observed a significant improvement with complete remission. MPS injection was given 40 mg 3 times for 10 days intravenously and Tablet Itraconazole 200 mg 1 time daily and Tablet AZT 50 mg with increasing dose to 100 mg 1 time daily.
During discharge, our schedule was Tablet AZT 200 mg 1 time daily for 3 months, Tablet MPS in tapering dose to 4 mg 1 time daily and AZT 100 mg 1 time daily. Additionally, we have offered Trimethoprim Sulfamethoxazole (160/800) with the dose of two times daily for one month and then one time for one month as an opportunistic infection’s prophylaxis after use of steroid and AZT. We have documented near-complete remission of skin lesions and lung lesions. After 3 months, MPS 4 mg alternate day daily with AZT 100 mg daily were continued for 1 year. We have documented complete remission of GPA maintained with AZT 100 1 time daily till 24 months.
DISCUSSION
Palpable purpura is the most common dermatological manifestations in GPA with a histopathology picture suggestive of leucocytoclastic vasculitis.[2] Characteristically, Churg-Strauss granuloma, extravascular necrotising granuloma of Winkelmann, palisaded neutrophilic and granulomatous dermatitis or interstitial granulomatous dermatitis typically develop around elbows and knees as papules documented in GPA.[3] Alternaria is a dematiaceous fungus ubiquitously found in nature and considered as opportunistic infection as it has natural tendency to affect immunosuppressed hosts.[4] Risk factors would be recipients of organ or stem cell transplant, patients receiving systemic steroids, haematological malignancies, human immunodeficiency virus, diabetes mellitus and Cushing syndrome.[5] Alternaria infection presents heterogeneously in different cases ranging from papules and nodules to non-healing ulcers, and in some cases as erythematosus infiltrating patches, verruciform lesions and persistent tumorous lesions.[5] The diagnosis of this fungal infection needs fungal examination and molecular analysis with specific primers apart from routine histopathological examination.[6] Various antifungals have been used for treatment of this fungal disease. At present, itraconazole is considered as best option due to its potency, cost effectiveness, better tolerability and ease of administration with negligible side effects. The recommended doses of itraconazole for management of cutaneous alternariosis is 200–600 mg daily for 3 months. Various other antifungals use has been documented in management of these fungal infections such as fluconazole, terbinafine, amphotericin B, voriconazole and posaconazole.[4,6]
CONCLUSION
Although skin lesions are frequently documented in GPA, cutaneous alternariosis is less frequently reported. As the number of patients under immunosuppressive drug therapy and their survival time increases, the risk of these cutaneous infections is also likely to increase
KOH mount with an expert microbiologist and trained in mycology will give quick results as observed in our case. Histopathology is the most sensitive and reliable test to diagnose
Fungal culture and identification of species by PCR is the most specific test to diagnose cutaneous alternariosis as documented in our case
Although immunosuppression is predominant cause for cutaneous alternariosis, immune dysregulation resulting from ongoing GPA or treatment options used to treat GPA such as steroids and AZT may predispose to this opportunistic fungal infection.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Granulomatosis with Polyangiitis. Ann Rheum Dis. 2022;81:315-20.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous Manifestations of Wegener's Granulomatosis: A Clinicopathologic Study of 17 Patients and Correlation to Antineutrophil Cytoplasmic Antibody Status. J Cutan Pathol. 2007;34:739-47.
- [CrossRef] [PubMed] [Google Scholar]
- Update from the Laboratory: Clinical Identification and Susceptibility Testing of Fungi and Trends in Antifungal Resistance. Infect Dis Clin North Am. 2016;30:13-35.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous Alternariosis Caused by Alternaria Infectoria: Three Cases in Kidney Transplant Patients. Healthcare. 2013;1:100-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous Infections by Dematiaceous Opportunistic Fungi: Diagnosis and Management in 11 Solid Organ Transplant Recipients. Mycoses. 2019;62:121-7.
- [CrossRef] [PubMed] [Google Scholar]
- Interstitial Granulomatous Dermatitis and Palisaded Neutrophilic Granulomatous Dermatitis. Cutis. 2018;101:E19-21.
- [Google Scholar]








