Translate this page into:
Low-Dose Naltrexone Therapy in Recalcitrant Hailey-Hailey Disease
*Corresponding author: Gurunath Babasaheb Gavali, Department of Dermatology, Dr. Panjabrao Deshmukh Memorial Medical College, Maharashtra, India. gurunathgavali09@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gavali GB, Khatri H, Agrawal SN. Low-Dose Naltrexone Therapy in Recalcitrant Hailey-Hailey Disease. Indian J Postgrad Dermatol. doi: 10.25259/IJPGD_113_2024
Abstract
Hailey-Hailey disease (HHD), also known as benign familial pemphigus, is a rare genodermatotic disorder characterized by painful blistering and erosions in intertriginous areas. Exacerbations are often triggered by sweating, minor trauma, and secondary infections, leading to significant morbidity and reduced quality of life. This case series presents three biopsy-proven recalcitrant HHD patients treated with low-dose naltrexone (LDN), an off-label, low-cost, and low-risk therapeutic option. The patients, who had failed multiple conventional treatments, were administered LDN hydrochloride starting at 3 mg/day and titrated to 6 mg/day over eight weeks. All three showed marked clinical improvement, including resolution of erosions and ulcerations, reduced pain, and decreased erythema, with sustained results over four months without flare-ups. While these findings suggest LDN may be an effective adjunct or alternative therapy for HHD, further research is needed to confirm its efficacy, long-term safety, and potential applicability in other calcium transport-related disorders.
Keywords
Benign familial pemphigus
Dilapidated brick wall appearance
Genodermatotic
Hailey-Hailey disease
Low-dose naltrexone
INTRODUCTION
Benign familial pemphigus, often known as Hailey-Hailey disease (HHD), is an uncommon genetic condition of the skin. The Hailey brothers initially described the disease in 1939.[1] It is a chronic and recurrent condition. The lesions usually start as flaccid blisters and rupture spontaneously with minimal trauma forming painful erosions over the intertriginous region. Sweating, trauma and infections can all cause flare-ups.[1,2] Managing patients can be difficult. Antibiotics, systemic and intralesional corticosteroids, botulinum toxin type A, laser treatments and systemic immunosuppression are among the various treatments that have been explored; each has had variable and inconsistent results and potential serious side effects.[1] Although low-dose naltrexone (LDN) has been widely promoted on various platforms as an anecdotal treatment for individuals with HHD, it was not until 2016 that one theoretical article was published in peer-reviewed medical literature.[3]
We are reporting that three recalcitrant HHD patients treated successfully with LDN.
CASE SERIES
The dose naltrexone is unavailable in the market, so the 50 mg tablet was crushed and dissolved in 50 mL of distilled water to form a solution with 1 mg/kg concentration which was stored in a sterile container. Patients started on 3 mg/day and titrated monthly as per need. The clinical response, adverse effects and subjective quality of life are monitored and assessed monthly. The study was conducted for 6 months. Clinical response was measured on the basis of the absence of new lesions, improvement in erythema, healing of involved areas and decrease in pain and discomfort. Thorough clinical examination with laboratory monitoring was done on a monthly basis. Treatment started in the outpatient department (OPD) after obtaining written consent.
Report of cases
Three cases of females aged 75 years, 63 years and 50 years with the history of erythematous, macerated and eroding plaques on the inframammary area, abdominal folds, groin and buttocks for 15 years, 30 years and 10 years, respectively, presented in OPD. Clinical examinations and routine investigations, including complete blood count, erythrocyte sedimentation rate, liver function tests, kidney function tests and serum electrolytes, were done and they were found to be within normal limits. The biopsy was also sent for histopathological examination, which was consistent with the HHD. Figure 1 shows the skin biopsy of the lesions. epidermis showing acantholysis with dilapidated brick wall appearance of stratum malphigi and Widespread acantholysis with dilapidated brick wall appearance of epidermis respectively.

- (a) Epidermis with acantholysis and dilapidated wall appearance of the stratum Malpighi. (b) Widespread acantholysis with dilapidated brick wall appearance of epidermis.
After failing topical corticosteroid and antifungal medications, the patient began using Naltrexone (3 mg/d). Further details of the treatments are in Table 1 for all the cases. All the patients sustained the results by the end of the 6th months of initiation of the therapy. Figure 2a shows the before treatment erosive lesion with greyish slough and maceration in the inframammary area. Figure 2b shows after treatment complete resolution of the lesion with PIH in the right inframammary area. Similarly, Figure 3a depicts before treatment initiation of hyperpigmented patches with maceration in the groin. Figure 3b shows after treatment complete resolution of the lesions of the left groin.
| Cases | Age (years) | Initial dose of naltrexone (mg/day) | Maximum dose required | Total duration | Response at 4th week | Complete clearance at and healing with PIH | Relapse (until 6th month) | Adverse effect |
|---|---|---|---|---|---|---|---|---|
| Case 1 | 75 | 3 | 6 mg/day at 8th week | 6 months | No development of new lesions, Reduction in pain and erythema | 8th week | NO | NO |
| Case 2 | 63 | 3 | 4.5 mg/day at 4th week | 6 months | Reduced itching, pain and erythema | 12th week | NO | NO |
| Case 3 | 50 | 3 | 4.5 mg/day at 4th week | 6 months | No development of new lesions, old lesion stared healing with reduction in erythema pain and discomfort | 6th week | NO | NO |
PIH: Post inflammatory hyperpigmentation
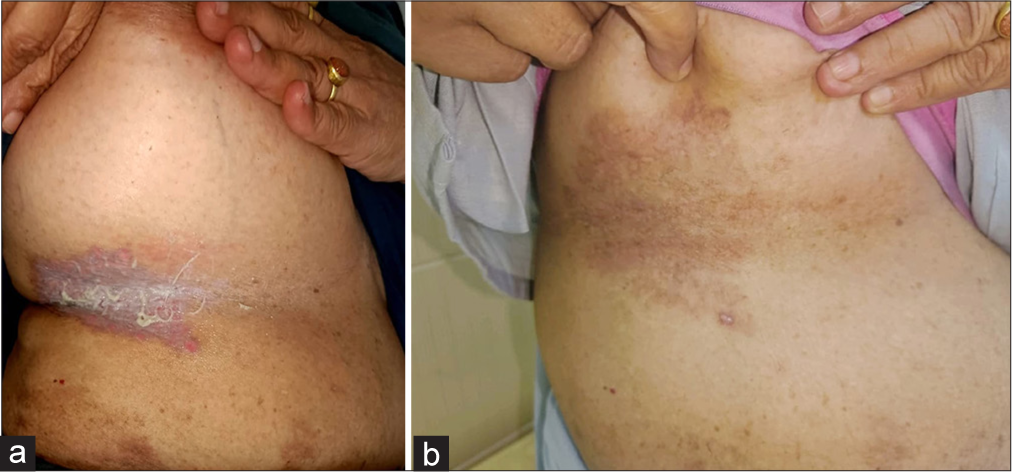
- (a) Before treatment erosive lesion with greyish slough and maceration in the inframammary area. (b) After treatment complete resolution of the lesion with PIH in the right inframammary area.
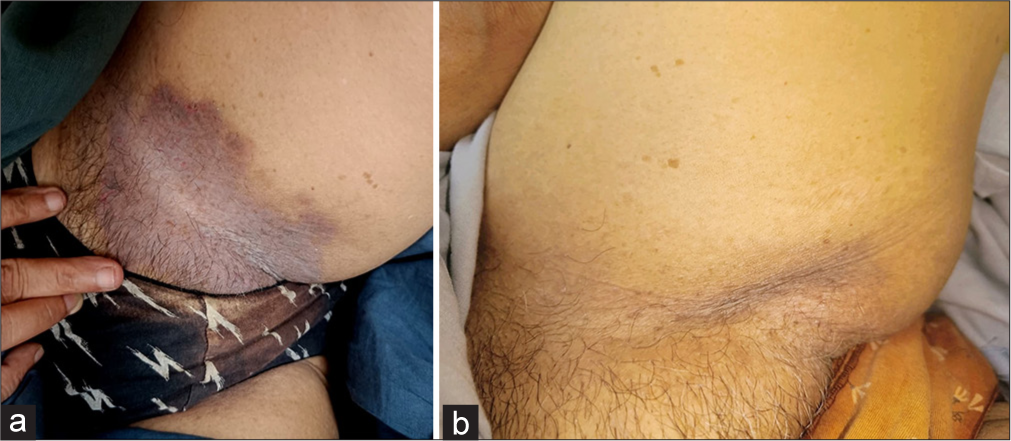
- (a) Before the initiation of treatment hyperpigmented patches with maceration in the groin. (b) After treatment complete resolution of the lesions of the left groin.
DISCUSSION
HHD is caused by a mutation in the ATP2 C1 gene on the third chromosome, which encodes the human secretory pathway Ca2+/Mn2+-ATPase isoform 1 of the Golgi apparatus.[4,5] A defective pump reduces calcium levels in the Golgi apparatus.[5] Cadherins, which are transmembrane glycoproteins found in desmosomes and adherens junctions, increase cell adhesion in a Ca2+-dependent way. The prevalence of HHD is approximately 1/50.000.[6]
The illness is frequently resistant to a variety of treatments, including topical and intralesional corticosteroids, retinoids, immunomodulators such as methotrexate and cyclosporine as well as physical and energy-based therapies. To the best of our knowledge, only a few studies have described LDN since 2016.
Naltrexone, an opioid receptor antagonist, was licensed by the Food and Drug Administration in 1984 for the treatment of opioid addiction at a dose of 50–100 mg/day. LDN has been tried for a variety of inflammatory conditions such as Crohn’s disease,[7] fibromyalgia,[8] complicated regional pain syndrome, chronic pruritus and multiple sclerosis.[9]
Opioid receptors from keratinocytes modulate many processes, including wound healing.[9] It could be possible that naltrexone may modulate μ receptors in the epidermis, with increased cellular adhesion and healing of the skin. Naltrexone also functions as a toll like receptor (TLR)-4 antagonist, and downstream signalling pathways are implicated in calcium homeostasis in keratinocytes.[10] Increased calcium allows the differentiation of keratinocytes and wound healing.
A full-dose naltrexone shows side effects such as suicidality, depression, hypersensitivity, headaches and vivid dreams, gastro intestinal (GIT) distress and hepatotoxicity, which are very rare with LDN. No organ system toxicities and abuse has been reported to date. Furthermore, there are no withdrawals with treatment discontinuation.[11]
LDN is a unique treatment for HHD since it has few side effects, improves symptoms quickly and significantly and is reasonably priced. Larger investigations are required to validate early findings and further investigate the process in HHD. LDN could be effective in various faulty calcium transport illnesses like Darrier Disease.
Table 2 depicts the review of the literature with LDN therapy in HHD.[12-16]
| Study by | Year | Conclusion |
|---|---|---|
| Albers et al.[12] | 2017 | Three cases with severe HHD improved completely within 3 months of therapy |
| Ibrahim, et al.[13] | 2017 | Three patients exhibited at least an 80% improvement with one patient reporting improvement in his depression |
| Riquelme-Mc Loughlin et al.[14] | 2019 | The study included 14 patients with a median age of 56.5 years. Six patients with no response, six with response and relapse, two shown sustained improvement for more than a year |
| Garayar Cantero et al.[15] | 2019 | Case report of 66-year-old women with complete clearance of the disease in 6 weeks of naltrexone 6.25 mg/day therapy initiation |
| Sousa Gomes et al.[16] | 2020 | A case of a 71-year-old white woman with vulvar HDD responded completely to low-dose naltrexone within 5 months. |
HHD: Hailey-Hailey disease
CONCLUSION
Three cases of HHD demonstrate the efficacy of LDN, with complete or near-total disease remission with sustained results by the 6th months. It is an off-label use, low-cost and low-risk option. Concomitant therapy can lead to greater improvements. The clinical evidence supporting its usage is quite preliminary, and more research is required.
Ethical approval:
The research/study approved by the Institutional Review Board at Dr.PDMMC Amravati, number PDMMC/SS/ETHICAL/6060/2024, dated 09 May 2024.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Laser Therapy for the Treatment of Hailey-Hailey Disease: A Systematic Review with Focus on Carbon Dioxide Laser Resurfacing. J Eur Acad Dermatol Venereol. 2015;29:1045-52.
- [CrossRef] [PubMed] [Google Scholar]
- Hailey-Hailey Disease: The Clinical Features, Response to Treatment and Prognosis. Br J Dermatol. 1992;126:275-82.
- [CrossRef] [PubMed] [Google Scholar]
- Hailey-Hailey Disease is Caused by Mutations in ATP2C1 Encoding a Novel Ca(2+) Pump. Hum Mol Genet. 2000;9:1131-40.
- [CrossRef] [PubMed] [Google Scholar]
- Mutations in ATP2C1, Encoding a Calcium Pump, Cause Hailey-Hailey Disease. Nat Genet. 2000;24:61-5.
- [CrossRef] [PubMed] [Google Scholar]
- Calcium Pump Disorders of the Skin. Am J Med Genet C Semin Med Genet. 2004;131C:20-31.
- [CrossRef] [PubMed] [Google Scholar]
- Low Dose Naltrexone for Induction of Remission in Crohn's Disease. Cochrane Database Syst Rev. 2018;4:CD010410.
- [CrossRef] [Google Scholar]
- Fibromyalgia Symptoms are Reduced by Low-Dose Naltrexone: A Pilot Study. Pain Med. 2009;10:663-72.
- [CrossRef] [PubMed] [Google Scholar]
- Activation of the δ-Opioid Receptor Promotes Cutaneous Wound Healing by Affecting Keratinocyte Intercellular Adhesion and Migration. Br J Pharmacol. 2015;172:501-14.
- [CrossRef] [PubMed] [Google Scholar]
- Human Keratinocytes Express Functional CD14 and Toll-Like Receptor 4. J Invest Dermatol. 2002;119:424-32.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of HaileyHailey Disease with Low-Dose Naltrexone. JAMA Dermatol. 2017;153:1018-20.
- [CrossRef] [PubMed] [Google Scholar]
- Low-Dose Naltrexone Treatment of Familial Benign Pemphigus (HaileyHailey Disease) JAMA Dermatol. 2017;153:1015-7.
- [CrossRef] [PubMed] [Google Scholar]
- Low-Dose Naltrexone Therapy in Benign Chronic Pemphigus (Hailey-Hailey Disease): A Case Series. J Am Acad Dermatol. 2019;81:644-6.
- [CrossRef] [PubMed] [Google Scholar]
- Use of Low-Dose Naltrexone in the Treatment of Severe Hailey-Hailey Disease: One Case Report. Dermatol Ther. 2019;32:e12892.
- [CrossRef] [PubMed] [Google Scholar]
- Vulvar Hailey-Hailey Disease Treated with Low-Dose Naltrexone: Case Report and Literature Review. Arch Gynecol Obstet. 2020;302:1081-6.
- [CrossRef] [PubMed] [Google Scholar]







