Translate this page into:
Diagnosis of Leprosy: Current Updates and Future Directions
*Corresponding author: Ananta Khurana, Department of Dermatology, Atal Bihari Vajpayee Institute of Medical Sciences and Dr. Ram Manohar Lohia Hospital, New Delhi, India. drananta2014@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bathula S, Khurana A, Singh I. Diagnosis of Leprosy: Current Updates and Future Directions. Indian J Postgrad Dermatol 2023;1:13-23.
Abstract
Leprosy is a chronic infectious granulomatous disorder caused by Mycobacterium leprae, chiefly affecting skin and peripheral nerves. It is the only known bacteria to infect nerves. Clinical diagnosis of leprosy is made when any of the three cardinal signs defined by the World Health Organisation is present. However, leprosy has varied presentations necessitating laboratory diagnostic methods for diagnosis as well as treatment initiation and monitoring. Slit-skin smears and histopathology form the basic diagnostics of maximum utility, while neurological studies, cytology and imaging have significant specific roles. Novel molecular and serological tests are of value in the diagnosis of early, indeterminate, and paucibacillary leprosy, and for screening of asymptomatic contacts. Molecular methods have in addition found an important place in diagnosis of drug resistance in leprosy.
Keywords
Leprosy
Diagnosis
Slit-skin smear
Histopathology
Molecular methods
Nerve conduction studies
INTRODUCTION
Leprosy, a chronic infection caused by Mycobacterium leprae, has particular predilection for peripheral nerves, skin and mucosa. The diagnosis of leprosy largely depends on the clinical presentation, especially in resource-constrained settings. Clinical diagnosis of leprosy is based on the World Health Organisation (WHO) criteria, which includes the presence of at least one of the three clinical signs of leprosy.[1]
Definite loss of sensation in a pale (hypopigmented) or reddish skin patch
A thickened or enlarged peripheral nerve with loss of sensation and/or weakness of the muscles supplied by that nerve
The presence of acid–fast bacilli in a slit-skin smear (SSS)
The various diagnostic methods used in leprosy are enlisted in Table 1 and detailed in further sections. SSS and histopathology remain the most commonly used laboratory methods in the diagnosis of leprosy.
| Test | Utility |
|---|---|
| Slit-skin smears | Diagnosis and classification of leprosy Treatment monitoring Identification of resistance Prediction of relapse |
| Histopathology | Diagnosis and spectrum determination Diagnosis of relapse |
| Nerve conduction studies | Diagnosis of pure neuritic leprosy |
| High-frequency ultrasonography | Diagnosis of pure neuritic leprosy, peripheral nerve involvement, reactions |
| Polymerase chain reaction | Diagnosis of leprosy (especially paucibacillary leprosy) Identification of drug resistant bacilli |
| Serology | Screening of asymptomatic contacts Prediction of leprosy reactions |
| Cytology | Subclassification of leprosy Diagnosis of pure neuritic leprosy |
SKIN SMEAR EXAMINATION
This is the most basic test in diagnostic algorithm of leprosy and gives information not only of the presence but also of the volume of infection in the patient, which has important therapeutic implications. M. leprae is an acid fast as well as alcohol fast bacteria. ‘Acid–fastness’ refers to the ability of bacilli to retain primary dye (carbol fuchsin) when treated with acid. Modified Ziehl–Neelsen (Z-N) method is used for staining of M. leprae, wherein mixture of acid-alcohol is mostly used for the decolourisation step [Figure 1].

- Staining kit used in Modified Ziehl–Neelsen staining for demonstration of acid–fast bacilli.
Procedure
The smear is taken while squeezing the skin to diminish the bleeding and incision (about 5 mm long and 3 mm deep) is given to collect tissue fluid. Precaution is to be taken to avoid blood in the collected material as it dilutes the number of bacilli in the smear. Thin smear of the material is prepared and heat fixed by passing the slide 3–4 times through the flame of a Bunsen burner. Carbol fuchsin (lipid soluble and penetrates the waxy cell wall) is poured over smear and underside of the slide is gently heated by passing a flame until fumes appear (without boiling). Overheating should be avoided and the stain is allowed to stand for 5 min. Smears are rinsed with water until no colour appears in the effluent. Sulphuric acid (5%) or acid-alcohol (1%) is poured over the slide and kept for one minute and this step is repeated until the slide appears light pink in colour (15–20 s). Smear is washed well with clean water, and then, the smears are covered with methylene blue or malachite green stain for 1–2 min. The stain is washed with clean water and slide is examined under microscope, using the ×100 oil immersion objective.
Interpretation of smears
The solid staining bacilli, representing living bacilli, appear as uniformly stained rods, whereas dead bacilli appear irregularly stained (fragmented bacilli) or as granules (granular bacilli). The density of bacilli (both living and dead) in smears is known as the bacteriological index (BI) and is expressed by Ridley’s logarithmic scale based on the number of bacilli seen in an average microscopic field using an oil-immersion objective [Figure 2]. Histoid lesions typically show longer appearing bacilli with tapered ends, which are not usually clumped as globi.

- Leprosy smears (Modified Ziehl -Neelsen staining). (a) BI 4+: 10–100 bacilli in a single oil immersion field, (b) BI 5+: 10–100 bacilli in a single oil immersion field, (c) BI 6+: globi (> 1000 bacilli) in a single oil immersion field, (d) Mycobacterium leprae highlighted on fluorescent staining, showing solid, fragmented, and granular bacilli (HiMedia® K021-1KT).
It requires about 104 bacilli/g of tissue for reliable detection by Z-N staining.[2,3] Thus, acid–fast bacilli (AFB) are absent in a typical tuberculoid (TT) lesion and either absent or scanty in borderline tuberculoid (BT) lesions. BI does not fall during first 12 months in lepromatous patients under treatment, as both dead and living bacilli are counted; however, it gradually declines at a rate of about 0.62 log/year thereafter and disappears over the next 5–10 years.
The percentage of solid staining bacilli is expressed as morphological index (MI), which is calculated after examining preferably 200 singly lying red staining elements. MI is a crude measure of viability of bacilli and is helpful in determining the activity of the disease.[4] Further, MI can be used to monitor response to treatment and an increase in MI on treatment suggests drug resistance or defaulter status. MI of lepromatous patients will be between 25% and 75% before initiation of multi-drug therapy (MDT) and there is a steady fall in MI to zero in 4–6 months of dapsone monotherapy, whereas it is considerably faster with MDT. However, there exists significant differences in MI depending on the different sites examines, with smears from nasal mucosa of lepromatous leprosy (LL) patients often revealing higher MI compared to those from skin and ear lobes.[5] Further, the higher MI may persist for longer in nasal mucosa and normal bacilli may reappear here but not elsewhere.
There are different guidelines regarding the number of skin smear sites and it has been changing over the years. Latest WHO recommendation is to prepare smears from a minimum of three sites (one ear lobe and two active lesions).[6] Two smears have to be taken from diametrically opposite active edge of the lesion when a single lesion is present. ILEP recommends use of two sites for initial smear (ear lobe and edge of most active area of an active looking lesion).[7]
The diagnostic specificity of SSS is 100% when a proper staining procedure is followed; however, recent studies have shown that SSS has a 5-year average sensitivity of 31.4%.[8,9] A study from India reported a SSS positivity of 100% in LL and histoid leprosy, 86.4% in borderline lepromatous (BL), 38.8% in BT and none in TT, indeterminate and pure neuritic leprosy (PNL).[2] The overall sensitivity of SSS was 59.8% in multibacillary (MB) and 1.8% in paucibacillary (PB) leprosy.[2]
HISTOPATHOLOGY
The preferred site for biopsy is the most active part of the lesion, which is usually at the periphery.[10-12] In patients where lesions of different spectrum are present, biopsy must be obtained from the most downgraded lesion to obtain most clinically useful information.[13] Most biopsies of leprosy irrespective of the spectrum show the leprosy pattern which is characterised by superficial and deep discrete, perivascular, periappendageal and perineural inflammatory infiltrate in an oval, oblong or curvilinear configuration.[12] Histopathologic features can be extremely useful in classifying the type of leprosy and identification of the presence of a leprosy reaction.
The presence of AFB within dermal nerves in a skin biopsy specimen is pathognomonic of leprosy. The diagnostic specificity of skin biopsy specimens and histopathologic examination ranges from 70% to 72%, but the sensitivity remains lower, ranging from 49% to 70%.[14] The dominant type of infiltrate present in the leprosy reaction pattern defines the spectrum of leprosy and this is detailed in [Table 2 and Figure 3], while histopathology of indeterminate leprosy and histoid leprosy is detailed below.
| TT | BT | BB | BL | LL | |
|---|---|---|---|---|---|
| Epidermal atrophy | Areas of atrophy+ | Variable | Atrophic | Atrophic | Thin and atrophic with complete flattening of rete ridges |
| Granulomas | Organised compact granulomas eroding epidermis | Epithelioid granulomas less compact than that of BT | Mixed cellular type (epithelioid cells and macrophages, epithelioid cells predominate) | Macrophage granuloma | Macrophage granuloma |
| Lymphocytes | +++++ | ++ | + | ++++ | +++ |
| Epithelioid cells | ++++ | +++ IEC++MEC absent |
++ | + | - |
| Giant cells | ++ (Langhan’s type) |
+++ (Foreign body type) |
- | - | - |
| Macrophages | - | + | ++ | +++ | +++++ |
| Grenz zone | Obliterated by granulomas | Present, granulomas touch epidermis at focal points | Clear grenz zone | Clear grenz zone | Clear grenz zone |
| Perineural lamination | - | - | + | + (concentric perineural cell proliferation, gives “onion peel” appearance |
+ (concentric perineural proliferation seen in subpolar LL) |
| AFB | 0 | 1+ | 2+−3+ | 3+−4+ | 5+−6+ |

- (a) Multiple compact epithelioid cell granulomas (*) predominantly composed of lymphocytes and foreign body giant cells with granulomas abutting epidermis at focal points suggestive of borderline tuberculoid leprosy (H&E, ×200), (b) multiple loosely formed granulomas (*) composed of lymphocytes and foam cells (black arrow) suggestive of borderline lepromatous leprosy (H&E, ×400), and (c) loosely formed granulomas (*) predominantly composed of macrophages, with sheets of foamy cells (black arrows) suggestive of lepromatous leprosy (H&E, × 400) (Image Courtesy: Dr. Kumari Ritu).
Indeterminate leprosy
AFBs may be seen occasionally in normal nerve, arrector pylori muscle, hair follicles, subepidermal zone and/or perivascular infiltrates in the early stage, whereas lymphocyte infiltration or Schwann cell proliferation characterise the late stage. Lymphocyte infiltration usually involves perineural sheath with preservation of nerve parenchyma, but the nerve fibre could be completely replaced by lymphocytes occasionally.[13] Proliferation of Schwann cells results in loss of wavy pattern of nerves and loss of longitudinal orientation of individual Schwann cell nuclei resulting in ‘baton’-shape of nuclei in normal nerves.
Histoid leprosy
Histopathology reveals hypercellular granuloma, predominantly composed of spindle-shaped cells. Pseudocapsule is seen as the centrifugal growth of these cells compresses the fibrous tissue. Solid staining bacilli are arranged in parallel stalks within the cells, referred to as histoid habitus. Islands of epithelioid cells without any organism inside may be seen in few histoid lesions, known as epithelioid contaminants.
Staining of AFBs on tissue
Different staining methods have been used for demonstration of AFBs in tissue samples. The density of the bacilli required to identify a single bacillus in the section by Fite-Faraco (FF) method is about 1000 per cubic millimetre of the tissue.[15] Fluorescent method has been found to be more sensitive than modified FF and ZN methods in detecting lepra bacilli in tissue sections especially when BI is less than three.[16] Sensitivity of fluorescent stain for indeterminate and BT leprosy was found to be 100%, and thus, this is most reliable in the categorisation of PB and MB leprosy (Figure 2).[16]
NOVEL DIAGNOSTIC METHODS IN LEPROSY
Molecular diagnosis of leprosy
While smear examination and histopathology enable precise diagnosis of the disease and its spectrum in most instances, additional diagnostic methods may be required in the following scenarios:
Early leprosy
Indeterminate leprosy
Paucibacillary leprosy
PNL
Early reactions
Asymptomatic contacts.
The definitive identification of M. leprae is possible through the extraction of nucleic acid, amplification and identification of M. leprae DNA in clinical specimens using polymerase chain reaction (PCR) [Flow chart 1]. Samples on which PCR can be performed include skin biopsy, skin smears, nerves, urine, oral or nasal swabs, blood and ocular lesions.[17] RLEP is the most sensitive genes for detecting M. leprae and RLEP and 16SrRNA are most commonly used.[18,19] Common genes employed in PCR assays to diagnose leprosy are listed in [Table 3].[20-29] PCR has facilitated the direct quantification of the bacterial DNA content in clinical samples, thereby increasing the reliability of the results.[20]
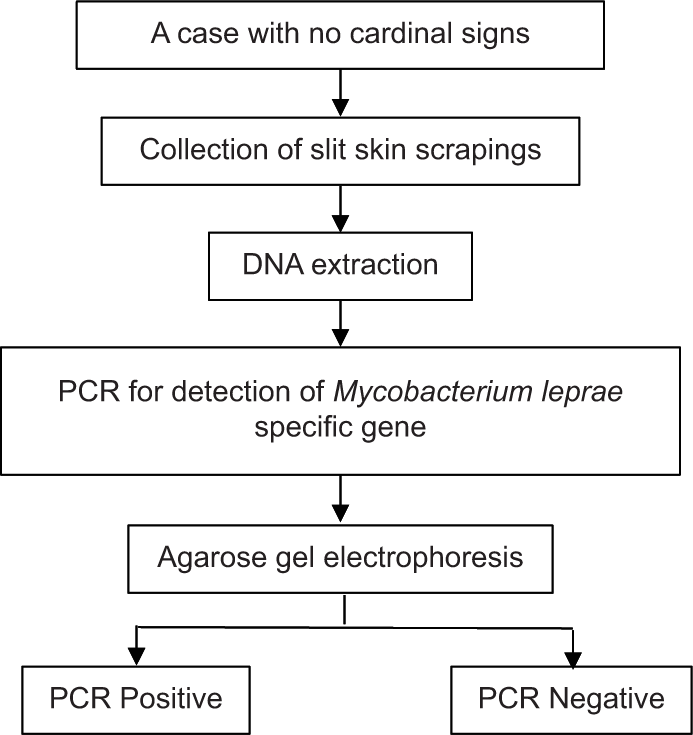
- PCR procedure in leprosy. PCR: Polymerase chain reaction.
| Gene targets | PCR method | Percentage positivity | References | |
|---|---|---|---|---|
| MB (%) | PB (%) | |||
| 36 kDa (PRA gene) | Real time PCR | 89 | 33 | Kramme et al. 2004 |
| 18 kDa | PCR | 99 | 74 | Williams et al. 1992 |
| RLEP | PCR | 100 | 73 | Yoon et al. 1993, Goulart et al. 2007, Turankar et al.2015, Pathak et al.2019 |
| RLEP+TTC | Multiplex PCR | 100 | 83 | Banerjee et al. 2010 |
| Ag85B | Real time PCR | 100 | 80 | Martinez et al. 2006 |
| 16S rRNA | Real Time PCR | 100 | 50 | Rudeeaneksin et al. 2008 |
| 16S rRNA | PCR | 70.96 | 31.25 | Katoch et al. 1994, Pathak et al. 2019 |
| SodA | PCR | 57 | 22.5 | Turankar et al. 2015, Pathak et al. 2019 |
| RLEP+16S rRNA +sodA | Multiplex PCR | 100 | 93 | Pathak et al. 2019 |
PCR: Polymerase chain reaction, MB: Multibacillary, PB: Paucibacillary
Quantitative PCR (qPCR) is emerging as the most suitable method for diagnosis of leprosy, particularly PB leprosy.[4] It is also useful in the identification of drug resistant bacilli, and this, in fact, has become an important use in leprosy in current times. Notably, it helps in differentiation of relapse from reaction by way of viability assays. Viability of bacilli can be detected by extraction of RNA from clinical samples, followed by complementary DNA synthesis and amplification by real time PCR. However, there are certain drawbacks as it has many targets and data on Good Manufacturing Practices products used for diagnostic purposes are lacking.
PCR sensitivity ranges from 30% to 83% in patients with a negative BI or with tuberculoid leprosy, while the sensitivity is 87–100% in those with a positive BI.[18,30] Recently, PCR has been developed by multiplexing of two or three genes specific for M. leprae to increase the sensitivity for diagnosing early leprosy cases/household contacts/patients with vague or no symptoms.[25,26]
Recent advance in molecular diagnosis of leprosy includes duplex-droplet digital PCR with greater sensitivity of detecting M. leprae DNA in PB patients compared with qPCR (79.5% vs. 36.4%), while both assays had a 100% sensitivity in MB patients.[31] Loop-mediated isothermal amplification is developed as a field friendly, cost-effective diagnostic tool and utilise RLEP and 16S rRNA gene targets to detect M. leprae.[32-34]
Drug resistance testing of M. leprae can be done using mouse foot pad inoculation as M. leprae is uncultivable in artificial media. However, it is time taking, cumbersome and expensive method. PCR followed by Sanger sequencing can be used for detection of drug resistant strains of M. leprae by targeting RpoB gene for rifampicin, FolP gene for dapsone and GyrA gene for ofloxacin resistance [Figure 4].

- Chromatogram showing point mutations in rpoB, folP, and gyrA gene followed by BLAST result.
Serology
Phenolic glycolipid 1 (PGL-1), a cell wall species-specific glycolipid, is the most widely used antigen for serological assays in leprosy.[35] The synthetic sugars – natural trisaccharide (NT) and natural disaccharide (ND) were synthesised and conjugated with either bovine serum albumin (BSA) or human serum albumin (HSA) using either octyl (O) or phenyl (P) linker arms (ND-O-BSA/HSA or NT-O-BSA/NT-P-BSA) as these showed higher affinity for IgM antibody than PGL-1. PGL-1 ELISA has 90–95% positivity for diagnosis of BL/LL cases and 25–60% positivity for diagnosis of TT/BT cases.[36] These antigens are also utilised in M. leprae dipstick assay and particle agglutination assay.[37] An immunochromatographic strip test is a quick output lateral flow assay for the detection of antibodies in field conditions and it takes only 10 min to perform.[38]
Recombinant 35kD protein can diagnose 40% of PB cases.[39] Leprosy Infectious Disease Research Institute Diagnostic 1 (LID-1) was found to be positive in 95% of MB cases and 20–40% of PB cases.[39] Antibody level against PGL-1, LID-1 and NDO-LID (synthetically conjugated LID-1 and ND-OBSA) was declined significantly after 6 month and 12 month of MDT treatment and, thus, can be used for monitoring treatment response. The major membrane proteins-I and II are the other antigens which have been used for serological diagnosis.[37,40-42] However, they seem to add a little value in the diagnosis.[43]
There are many studies that stress about high risk of developing leprosy among household contacts with positive anti-PGL-I titres. However, the role of positive anti-PGL-1 titres in the detection of preclinical leprosy among household contacts of leprosy patients may not be applicable in endemic areas as many individuals with seropositivity will never develop leprosy. In fact, it has been shown that more than half of the individuals with antibodies against PGL-1 will never develop leprosy.[44,45]
Studies have been conducted to analyse the utility of serological tests in the prediction of reactions in leprosy patients. A recent study found higher anti-LID-1 levels in patients with type 2 reaction (T2R) at diagnosis compared to type 1 reaction (P = 0.008) and non-reactional patients (P = 0.020). The author concluded that high and persistent anti-LID-1 antibody levels in MB leprosy might be a useful tool to predict susceptibility of patients to develop T2R.[45] Similar findings were later reported by Devides et al. using anti-PGL-1 and anti-NDO-LID-1 levels.[46]
Cytology in leprosy
Singh et al. have suggested cytology criteria for subclassification of leprosy.[47] Samples for cytology may be from skin lesions, nerve or lymph nodes. Cytology demonstrates cohesive epithelioid cell granulomas with lymphocytes, not infiltrating the granuloma, in tuberculoid leprosy.[47] The cohesion between the cells of the granulomas declines, with concurrent increase in infiltration of lymphocytes within them, as the disease downgrades toward the lepromatous pole.
Important use of cytology lies in diagnosis of pure neural leprosy where tissue for histopathology is difficult to obtain, as even motor and mixed nerves can be safely sampled for cytology.[4] Further, the aspirate can be used for PCR detection of M. leprae as well, enhancing diagnostic yield in PB spectrum.
PURE NEURITIC LEPROSY (PNL)
PNL is defined as exclusive nerve involvement in the form of nerve thickening or neural deficit without any skin lesions and a negative SSS, in the absence of other causes of nerve involvement.[48,49] PNL poses a diagnostic challenge mainly due to unavailability of tissue amenable to smear and histopathological examination. Nerve involvement is mostly in the form of mononeuritis (approximately 60%).[50] However, mononeuritis multiplex and polyneuritic form, also called ‘mononeuritis multiplex summation,’ are also not uncommon and if present, should lead to thorough evaluation to rule out LL.[51,52] Skin smears should thus be done in of the latter scenario.
Nerve biopsy
Nerve biopsy is considered as gold standard for the diagnosis of PNL (as skin lesions are absent). A thickened sensory nerve which lacks motor fibres such as a supraorbital branch of the 5th cranial nerve, a supraclavicular nerve, the great auricular nerve in the neck, the radial nerve at the wrist, a cutaneous nerve of forearm or thigh, the sural nerve behind the lateral malleolus or a superficial peroneal nerve on the dorsum of the foot are considered suitable for nerve biopsy [Figure 5]. We mostly perform sural nerve biopsy at our centre due to ease of isolation, ability to obtain sufficient sample for biopsy (considering the bulk of the nerve) and as sensory loss in area of distribution of sural nerve is less concerning to patients than sensory loss following biopsy of radial cutaneous nerve.

- (a and b) Marking and dissection of sural nerve in nerve biopsy (Image: Khurana A. Diagnosis of leprosy. In: Sardana K, Khurana A, editors. Jopling’s Handbook of Leprosy. 7th ed. New Delhi: CBS publishers; 2023).
Nerve conduction studies
NCS in nerves affected by leprosy may show reduced amplitude of sensory nerve action potentials and compound muscle action potential or CMAP suggestive of axonal damage, and decreased nerve conduction velocity and increased latency due to demyelination.[53] A prospective study found that 100% MB and 50% PB leprosy cases showed abnormalities on NCS at the time of diagnosis of leprosy.[54]
Studies demonstrate early detection of nerve dysfunction by NCS, before the appearance of typical symptoms and signs of nerve function impairment, thus helping in detecting subclinical neural involvement, although the therapeutic implication of this is yet undetermined.[55] Interestingly, a recent study comparing combination of nerve palpation with Semmes-Weinstein monofilament testing and voluntary muscle testing showed comparable efficacy to NCS in detecting nerve damage.[56]
High-frequency ultrasonography
High-frequency ultrasonography (15–20 MHz) helps in better identification of nerves and gives details about features such as exact site and size of nerve thickness, morphological variations in nerve trunk such as texture, pattern of fascicles and vascularity.[55] Loss and destruction of fascicular pattern is the most specific feature for neural impairment in leprosy.[56] It is particularly important in the diagnosis of PNL and is most useful for the assessment of nerves that are inaccessible for clinical palpation, such as the median nerve at the wrist; however, higher sensitivity and specificity have been reported for ulnar and common fibular nerves. Reactions in PNL shows increased vascularity and oedema of nerve trunk, suggestive of neuritis.
The cross-sectional area (CSA) of peripheral nerves is an important tool in the detection of large areas of nerve damage in leprosy. Frade et al. have suggested the measurement of the asymmetry index in the evaluation of leprosy and have demonstrated that this index is highly sensitive and specific for the differentiation between the nerves of healthy individuals and the nerves of patients with leprosy.[56] The ROC analysis of CSAs showed the best specificity and sensitivity at the pre-tunnel (PT point) of the ulnar and common fibular nerves, respectively. Leprous neuropathy shows an increased CSA and the pattern of asymmetry (ΔCSA>2.5 mm2 with an RR of 13) with high sensitivity and specificity for its early diagnosis.[57]
Some older test, not in common use in current practice, are detailed in [Table 4].[4]
| Test | ||
|---|---|---|
| Lepromin test | • It is a delayed type hypersensitivity reaction to M. leprae or its antigens and has limited practical use as it doesn’t indicate exposure. • Previously used for classifying leprosy. It is assessed by the intradermal injection of 0.1 mL of lepromin, a suspension of heat-killed M. leprae, obtained from experimentally infected armadillos. |
The response is evaluated by measuring the diameter of induration at the injection site at 2 days (Fernandez reaction) and at 3–4 weeks post-inoculation (Mitsuda reaction). |
| Sweating test | • The test is carried out to assess integrity of dermal nerves. • It involves intradermal injection of 0.2 mL of a 1 in 1000 solution of pilocarpine nitrate into the lesion to be tested, the area is painted with tincture of iodine and then dusted with starch powder. |
Sweating causes blue discoloration of the powder, whereas there it is absent if there is anhidrosis due to damage to dermal nerve. Anhidrosis is characteristic feature of tuberculoid leprosy. |
| Histamine test | Histamine can be used to test the damage integrity of dermal nerves and degree of damage to these nerves can be measured by the reduction in size and brightness of the histamine flare. This assists in deciding if a hypopigmented macule is due to leprosy. | The flare is delayed in a leprosy macule, feeble in indeterminate and borderline leprosy or entirely absent in tuberculoid leprosy. |
M. leprae: Mycobacterium leprae.*Adapted from Khurana A. Diagnosis of leprosy. In: Sardana K, Khurana A, editors. Jopling’s Handbook of Leprosy. 7thed.. New Delhi: CBS publishers; 2023. p. 97-119
FUTURE DIRECTIONS
There is a need of improved accessibility of molecular methods, for both diagnosis and drug resistance testing, in endemic areas. Rapid point of care (POC) tests to enable accurate diagnosis in field settings is being investigated. Lateral flow assays based on finger-stick blood could provide a means for POC testing infection by measuring both antibodies and cytokines/chemokines in capillary blood.
Diagnostic methods which screen high-risk population and help in predicting the development of leprosy in susceptible individuals would be extremely useful in elimination of leprosy (defined now as no new autochthonous cases as a result of interruption of transmission) and achieving the goal of Zero Leprosy by enabling more effective and cost-efficient use of chemoprophylactic and immunoprophylactic measures.[58,59] Further, biomarkers for neural involvement and for diagnosis and follow-up of reactional cases are urgently required to prevent the disabilities resulting from leprosy.
Declaration of patient consent
Patient’s consent not required as the patient’s identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
MULTIPLE CHOICE QUESTIONS
Q1. All of the following statements are true except
Morphological index represents both living and dead bacilli in a smear MI can be used to monitor response to treatment MI is calculated after examining 200 singly lying solid bacilli in a smear MI of lepromatous patients will be between 25% and 75% before initiation of multi-drug therapy
Q2. Onion peel appearance is seen in
Tuberculoid leprosy Borderline lepromatous leprosy Lepromatous leprosy Indeterminate leprosy
Q3. Which of the following statement is true about polymerase chain reaction in leprosy?
PCR is more useful in diagnosis of MB leprosy compared to PB leprosy PRA and soda genes are most commonly employed in PCR assays to diagnose leprosy Sensitivity of PCR ranges from 20% to 30% in leprosy patients with positive bacteriological index Drug resistant bacilli can be detected using PCR technique
Q4. Maximum lymphocytes on histopathology are seen in
Borderline tuberculoid leprosy Primary tuberculoid leprosy Lepromatous leprosy Borderline lepromatous leprosy
Q5. All of the following are features of pure neuritic leprosy on nerve conduction studies except
Increased latency Decreased amplitude of action potentials Prolonged H reflex latency Decreased nerve conduction velocity
Q6. Which of the following tests has highest sensitivity in the diagnosis of leprosy?
Quantitative PCR ML flow test ELISA Agglutination assay
Q7. It requires about 100 bacilli/g of tissue for reliable detection of acid–fast bacilli by Z-N staining.
True False
Q8. Which of the following histopathological features is not seen in lepromatous leprosy?
Clear grenz zone Foamy macrophages Atrophic epidermis Langhans giant cells
Q9. False about lepromin test is
Helps in classifying leprosy Fernandez reaction is read at 48–72 h Type 3 hypersensitivity reaction to Mycobacterium leprae or its antigens No role in diagnosis of leprosy
Q10. Anti-PGL-1 is used in serological assays of leprosy. Which of the following is not true about this assay?
Anti-PGL-1 antibody levels increases from tuberculoid to lepromatous pole Positive titres in household contacts of leprosy patients in non-endemic areas could denote high risk of developing leprosy It is a very sensitive method to diagnose paucibacillary leprosy Higher and persistent levels might be a useful tool to predict susceptibility to type 2 leprosy reaction
Answers
a b d b c a b d c c
Financial support and sponsorship
Nil.
References
- Treatment and Prevention of Leprosy. Available from: https://www.who.int/publications/i/item/9789290226383 [Last accessed on 2022 Oct 27]
- [Google Scholar]
- Diagnosing leprosy: Revisiting the role of the slit skin smear with critical analysis of the applicability of polymerase chain reaction in diagnosis. Int J Dermatol. 2011;50:1522-7.
- [CrossRef] [PubMed] [Google Scholar]
- A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968;36:78-82.
- [Google Scholar]
- Diagnosis of leprosy In: Sardana K, Khurana A, eds. Jopling's Handbook of Leprosy (7th ed). New Delhi: CBS Publishers; 2023. p. :97-119.
- [Google Scholar]
- Some observations on the morphological index in lepromatous leprosy. Lepr Rev. 1966;37:23-5.
- [CrossRef] [PubMed] [Google Scholar]
- Available from: https://www.ilepfederation.org/wp-content/uploads/2016/11/how-to-do-a-smear-examination-forleprosynew-logo.pdf [Last accessed on 2022 Dec 05]
- An update on the diagnosis and treatment of leprosy. J Am Acad Dermatol. 2004;51:417-26.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitivity of slit skin smear examination in suspected leprosy cases in a tertiary care centre: Rising trends. Int J Sci Res. 2017;69:34-5.
- [Google Scholar]
- Pathology of leprosy In: Hastings RC, ed. Leprosy Vol 2. (2nd ed). Edinburgh: Churchill Livingstone; 1994. p. :193-224.
- [Google Scholar]
- Classification In: Ridley DS, ed. Pathogenesis of Leprosy and Related Diseases. Vol 15. UK: Butterworth and Co-publishers Ltd; 1988. p. :155-75.
- [CrossRef] [Google Scholar]
- The disease In: Jopling WH, McDougall AC, eds. Handbook of Leprosy (5th ed). New Delhi: CBS Publishers and Distributors; 1996. p. :10-53.
- [Google Scholar]
- Histopathology of leprosy In: Sardana K, Khurana A, eds. Jopling's Handbook of Leprosy (7th ed). New Delhi: CBS Publishers; 2023. p. :120-52.
- [Google Scholar]
- Bacterial diseases In: Elder DE, Elenitsas R, Rosenbach M, Murphy GE, Rubin AI, Xu X, eds. Lever's Histopathology of Skin (4th ed). Philadelphia, PA: Wolters Kluwer; 2015. p. :663-72.
- [Google Scholar]
- Evaluation of fluorescent staining for diagnosis of leprosy and its impact on grading of the disease: Comparison with conventional staining. J Clin Diagn Res. 2016;10:EC23-6.
- [CrossRef] [PubMed] [Google Scholar]
- Use of PCR-mediated amplification of Mycobacterium leprae DNA in different types of clinical samples for the diagnosis of leprosy. J Med Microbiol. 1993;39:298-304.
- [CrossRef] [PubMed] [Google Scholar]
- PCR-based techniques for leprosy diagnosis: From the laboratory to the clinic. PLoS Negl Trop Dis. 2014;8:e2655.
- [CrossRef] [PubMed] [Google Scholar]
- Application of RLEP real-time PCR for detection of M. leprae DNA in paraffin-embedded skin biopsy specimens for diagnosis of paucibacillary leprosy. Am J Trop Med Hyg. 2014;90:524-9.
- [CrossRef] [PubMed] [Google Scholar]
- Detection and quantification of Mycobacterium leprae in tissue samples by real-time PCR. Med Microbiol Immunol. 2004;193:189-93.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of Mycobacterium leprae and the potential for monitoring antileprosy drug therapy directly from skin biopsies by PCR. Mol Cell Probes. 1992;6:401-10.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of polymerase chain reaction amplification of Mycobacterium leprae-specific repetitive sequence in biopsy specimens from leprosy patients. J Clin Microbiol. 1993;31:895-9.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of Mycobacterium leprae DNA in skin lesions of leprosy patients by PCR may be affected by amplicon size. Arch Dermatol Res. 2007;299:267-71.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative evaluation of PCR amplification of RLEP, 16S rRNA, rpoT and Sod A gene targets for detection of M. leprae DNA from clinical and environmental samples. Int J Mycobacteriol. 2015;4:54-9.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of multiplex PCR for early diagnosis and household contact surveillance for leprosy. Diagn Microbiol Infect Dis. 2019;95:114855.
- [CrossRef] [PubMed] [Google Scholar]
- Multiplex PCR technique could be an alternative approach for early detection of leprosy among close contacts--a pilot study from India. BMC Infect Dis. 2010;10:252.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of real-time and conventional PCR targeting complex 85 genes for detection of Mycobacterium leprae DNA in skin biopsy samples from patients diagnosed with leprosy. J Clin Microbiol. 2006;44:3154-9.
- [CrossRef] [PubMed] [Google Scholar]
- LightCycler real-time PCR for rapid detection and quantitation of Mycobacterium leprae in skin specimens. FEMS Immunol Med Microbiol. 2008;54:263-70.
- [CrossRef] [PubMed] [Google Scholar]
- Progress in developing ribosomal RNA and rRNA gene(s) based probes for diagnosis and epidemiology of infectious disease specially leprosy In: Kumar S, Sen AK, Dutta GP, eds. Tropical Disease, Molecular Biology and Control Strategies. New Delhi: Council of Scientific and Industrial Research; 1994. p. :580-7.
- [Google Scholar]
- Comparison of PCR mediated amplification of DNA and the classical methods for detection of Mycobacterium leprae in different types of clinical samples in leprosy patients and contacts. Lepr Rev. 2003;74:18-30.
- [CrossRef] [PubMed] [Google Scholar]
- Development and evaluation of a droplet digital PCR assay for the diagnosis of paucibacillary leprosy in skin biopsy specimens. PLoS Negl Trop Dis. 2019;13:e0007284.
- [CrossRef] [PubMed] [Google Scholar]
- Development of a novel loop-mediated isothermal amplifcation assay for rapid detection of Mycobacterium leprae in clinical samples. Indian J Dermatol Venereol Leprol. 2021;87:491-7.
- [CrossRef] [Google Scholar]
- Development of a Loop-mediated isothermal amplifcation (LAMP) technique for specifc and early detection of Mycobacterium leprae in clinical samples. Sci Rep. 2021;11:9859.
- [CrossRef] [PubMed] [Google Scholar]
- Loop-mediated isothermal amplifcation (LAMP) assay targeting RLEP for detection of Mycobacterium leprae in leprosy patients. Int J Infect Dis. 2021;107:145-52.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence for species-specific lipid antigens in Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 1980;48:382-7.
- [Google Scholar]
- Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect Immun. 1983;41:1077-83.
- [CrossRef] [PubMed] [Google Scholar]
- Recent laboratory advances in diagnostics and monitoring response to treatment in leprosy. Indian Dermatol Online J. 2019;10:106-14.
- [CrossRef] [PubMed] [Google Scholar]
- Simple and fast lateral flow test for classification of leprosy patients and identification of contacts with high risk of developing leprosy. J Clin Microbiol. 2003;41:1991-5.
- [CrossRef] [PubMed] [Google Scholar]
- Serological survey of leprosy and control subjects by a monoclonal antibody-based immunoassay. Int J Lepr Other Mycobact Dis. 1985;53:33-8.
- [Google Scholar]
- Chemical synthesis of the trisaccharide unit of the species-specific phenolic glycolipid from Mycobacterium leprae. Carbohydr Res. 1987;163:41-52.
- [CrossRef] [PubMed] [Google Scholar]
- The allyl group for protection in carbohydrate chemistry. 17. Synthesis of propyl O-(3,6-di-O-methyl-beta-D-glucopyranosyl)-(1-4)-O-(2,3-di-O-methyl-alphaL-rhamnopyranosyl)-(1-2)-3-O-methyl-alpha-L-rhamnopyranoside: The oligosaccharide portion of the major serologically active glycolipid from Mycobacterium leprae. Chem Phys Lipids. 1985;38:299-307.
- [CrossRef] [PubMed] [Google Scholar]
- Position statement: LEPROSY: Diagnosis, treatment and follow-up. J Eur Acad Dermatol Venereol. 2019;33:1205-13.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective study of serological conversion as a risk factor for development of leprosy among household contacts. Clin Diagn Lab Immunol. 2004;11:897-900.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-PGL-1 positivity as a risk marker for the development of leprosy among contacts of leprosy cases: Systematic review and meta analysis. PLoS Negl Trop Dis. 2016;10:e0004703.
- [CrossRef] [PubMed] [Google Scholar]
- Multibacillary leprosy patients with high and persistent serum antibodies to leprosy IDRI diagnostic-1/LID-1: Higher susceptibility to develop Type 2 reactions. Mem Inst Oswaldo Cruz. 2015;110:914-20.
- [CrossRef] [PubMed] [Google Scholar]
- Can antiPGL-1 and anti-NDOLID-1 antibody titers be used to predict the risk of reactions in leprosy patients? Diagn Microbiol Infect Dis. 2018;91:260-5.
- [CrossRef] [PubMed] [Google Scholar]
- Cytomorphology of leprosy across the Ridley-Jopling spectrum. Acta Cytol. 1996;40:719-23.
- [CrossRef] [PubMed] [Google Scholar]
- Pure neural leprosy In: Sardana K, Khurana A, eds. Jopling's Handbook of Leprosy (7th ed). New Delhi: CBS Publishers; 2023. p. :85-92.
- [Google Scholar]
- Leprosy neuropathy: Clinical presentations. Arq Neuropsiquiatr. 2013;71:661-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical, electroneuromyographic and morphological studies of pure neural leprosy in a Brazilian referral centre. Lepr Rev. 2004;75:242-53.
- [Google Scholar]
- Pure neuritic leprosy: Current status and relevance. Indian J Dermatol Venereol Leprol. 2016;82:252-61.
- [CrossRef] [PubMed] [Google Scholar]
- Pure or primary neuritic leprosy (PNL) Lepr Rev. 2016;87:450-5.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitivity and specificity of nerve palpation, monofilament testing and voluntary muscle testing in detecting peripheral nerve abnormality, using nerve conduction studies as gold standard; a study in 357 patients. Lepr Rev. 2009;80:34-50.
- [CrossRef] [PubMed] [Google Scholar]
- Progression of leprosy neuropathy: A case series study. Brain Behav. 2012;2:249-55.
- [CrossRef] [PubMed] [Google Scholar]
- High-resolution sonography: A new technique to detect nerve damage in leprosy. PLoS Negl Trop Dis. 2009;3:e498.
- [CrossRef] [PubMed] [Google Scholar]
- New sonographic measures of peripheral nerves: A tool for the diagnosis of peripheral nerve involvement in leprosy. Mem Inst Oswaldo Cruz. 2013;108:257-62.
- [CrossRef] [PubMed] [Google Scholar]
- Point-of-care ultrasound of peripheral nerves in the diagnosis of Hansen's disease neuropathy. Front Med (Lausanne). 2022;9:985252.
- [CrossRef] [PubMed] [Google Scholar]
- Ending the Neglect to attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021-2030 Geneva: World Health Organization; 2020.
- [Google Scholar]
- Towards Zero Leprosy. Global Leprosy (Hansen's Disease) Strategy 2021-2030. Available from: https://www.who.int/publications/i/item/9789290228509 [Last accessed on 2022 Dec 07]
- [Google Scholar]







