Translate this page into:
TAXANE-Induced Cutaneous Manifestations
*Corresponding author: Suruthi Purushothaman, Department of Dermatology, Jawaharlal Institue of Postgraduate Medical Education and Reseach, Puducherry, India. suruthidvlpub@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vageshappa RK, Purushothaman S. TAXANE-Induced Cutaneous Manifestations. Indian J Postgrad Dermatol. doi: 10.25259/IJPGD_257_2024
Dear Editor,
A 30-year-old female came to our clinic presenting with asymptomatic linear hyperpigmentation on her left forearm, which developed following a peripheral intravenous injection. She had been diagnosed with the right breast carcinoma, confirmed as Her2nu positive and underwent a modified radical mastectomy. Subsequently, she received chemotherapy that included docetaxel, carboplatin and trastuzumab, with docetaxel administered through intravenous infusion over five cycles. Approximately 4 weeks after her first dose of docetaxel, she observed pigmentation at the injection site on her left forearm.
On clinical examination, linear streaks of hyperpigmentation were noted over the flexor side of her left forearm [Figure 1]. The veins beneath these pigmented streaks were neither tender nor thickened.
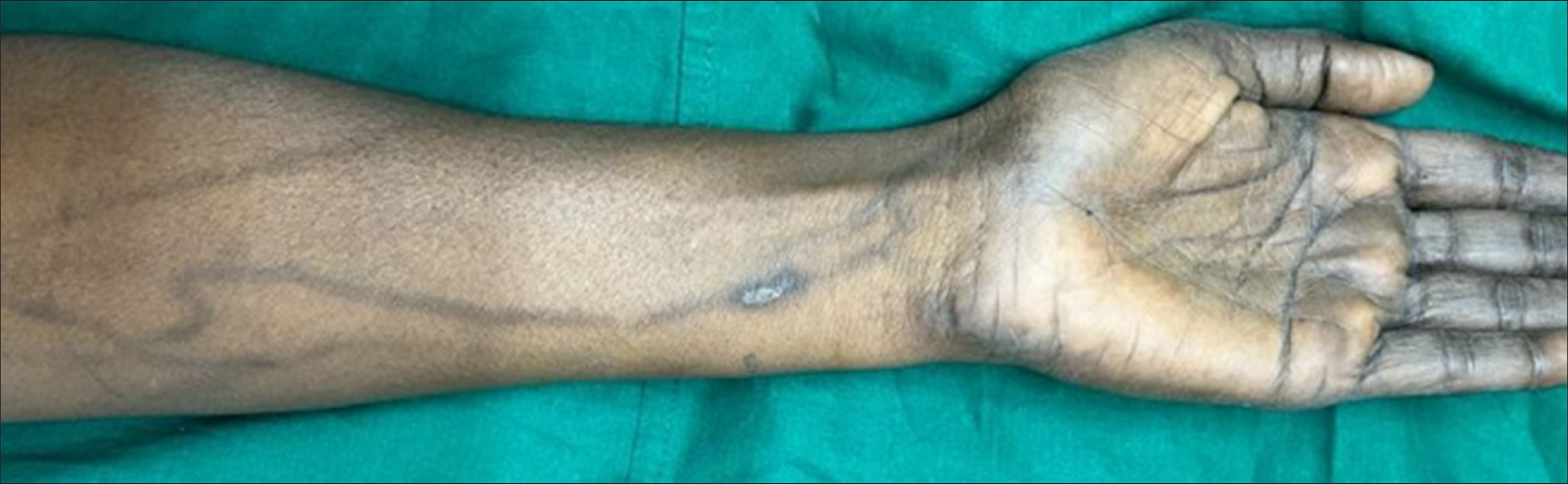
- Linear streaks of hyperpigmentation along the course of veins over the flexor aspect of the left forearm.
In addition, examination of her palms revealed ill-defined hyperpigmentation, scaling and increased pigmentation in the skin creases [Figure 2a]. All her fingernails exhibited bands of pigmentation [Figure 2b]. Chemotherapeutic agents are increasingly utilised in the treatment of breast cancer, yet dermatological side effects are often under-reported. Among the drugs known to cause cutaneous adverse effects, taxanes (such as Docetaxel and Paclitaxel) are the most commonly implicated.[1] Serpentine supra-venous hyperpigmentation is a rarely documented dermatological side effect associated with the combination of docetaxel and carboplatin.
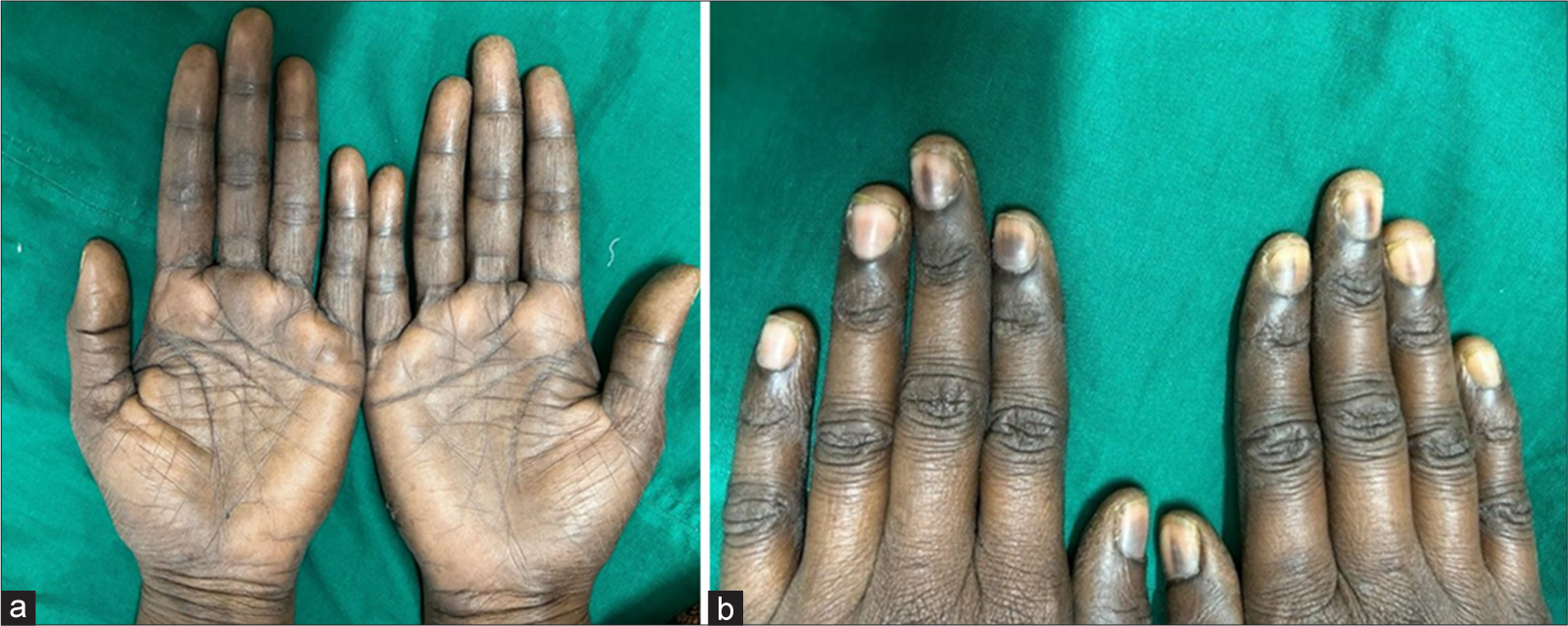
- (a) Diffuse hyperpigmentation of the palms with scaling and increased pigmentation of the creases. (b) All the fingernails show bands of pigmentation.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Dermatological Adverse Events with Taxane Chemotherapy. Eur J Dermatol. 2016;26:427-43.
- [CrossRef] [PubMed] [Google Scholar]






