Translate this page into:
Non-Familial and CYLD Gene Mutation Negative Brooke–Spiegler Syndrome Associated with Human Immunodeficiency Virus: A Case Report
*Corresponding author: Navya Pandey, Department of Dermatology, Krishna Vishwa Vidyapeeth, Karad, Maharashtra, India. navya24pan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pandey N, Nikam B, Jamale V, Hussain AA, Bhale G. Non-Familial and CYLD Gene Mutation Negative Brooke– Spiegler Syndrome Associated with Human Immunodeficiency Virus: A Case Report. Indian J Postgrad Dermatol. 2025;3:33-5. doi: 10.25259/IJPGD_63_2024
Abstract
Brooke–Spiegler syndrome is a rare autosomal dominant disorder characterised by the presence of various adnexal tumours including multiple cylindromas, trichoepitheliomas and spiradenomas. This is a case of a 52-year-old female, on antiretroviral therapy who presented with numerous painless nodules over her forehead, scalp and face for 3 years. The asymptomatic skin-coloured nodules extended from the forehead to the post-auricular region and measured 2–6 cm in size. Histopathology of the larger lesion showed cylindroma, the medium-sized lesion showed spiradenoma and the smaller skin-coloured papules showed trichoepithelioma. There was no history of similar lesions in the family and no other symptoms suggestive of any systemic involvement.
Keywords
Brooke–Spiegler syndrome
Cylindromatosis
Human immunodeficiency virus
Rare
INTRODUCTION
Brooke–Spiegler syndrome (BSS) is a rare disorder characterised by adnexal tumours, including cylindromas, trichoepitheliomas and spiradenomas.[1,2] We present a rare case of BSS in an human immunodeficiency virus (HIV)-positive patient on antiretroviral therapy, without a CYLD gene mutation or family history of the condition.
CASE REPORT
A 52-year-old female presented to the outpatient department with multiple asymptomatic nodules that began on her forehead, gradually increased in size and eventually involved the earlobes and post-auricular area over 3 years.[3] She was diagnosed with HIV and has been on antiretroviral therapy since 2007.
Cutaneous examination revealed multiple firm, papulonodular lesions with a smooth surface, ranging from 2 to 6 cm in size, over the bilateral earlobes and post-auricular area [Figure 1a]. Dermoscopy of a cylindroma showed multiple arborizing telangiectasias branching toward the periphery against a pink salmon background [Figure 1b]. Biopsy of the nodules revealed basophilic nodules with a fibrous capsule containing epithelial cells in intertwining cords. The peripheral cells had dark nuclei, while the central cells had large, pale nuclei surrounding the lumina [Figure 1c].
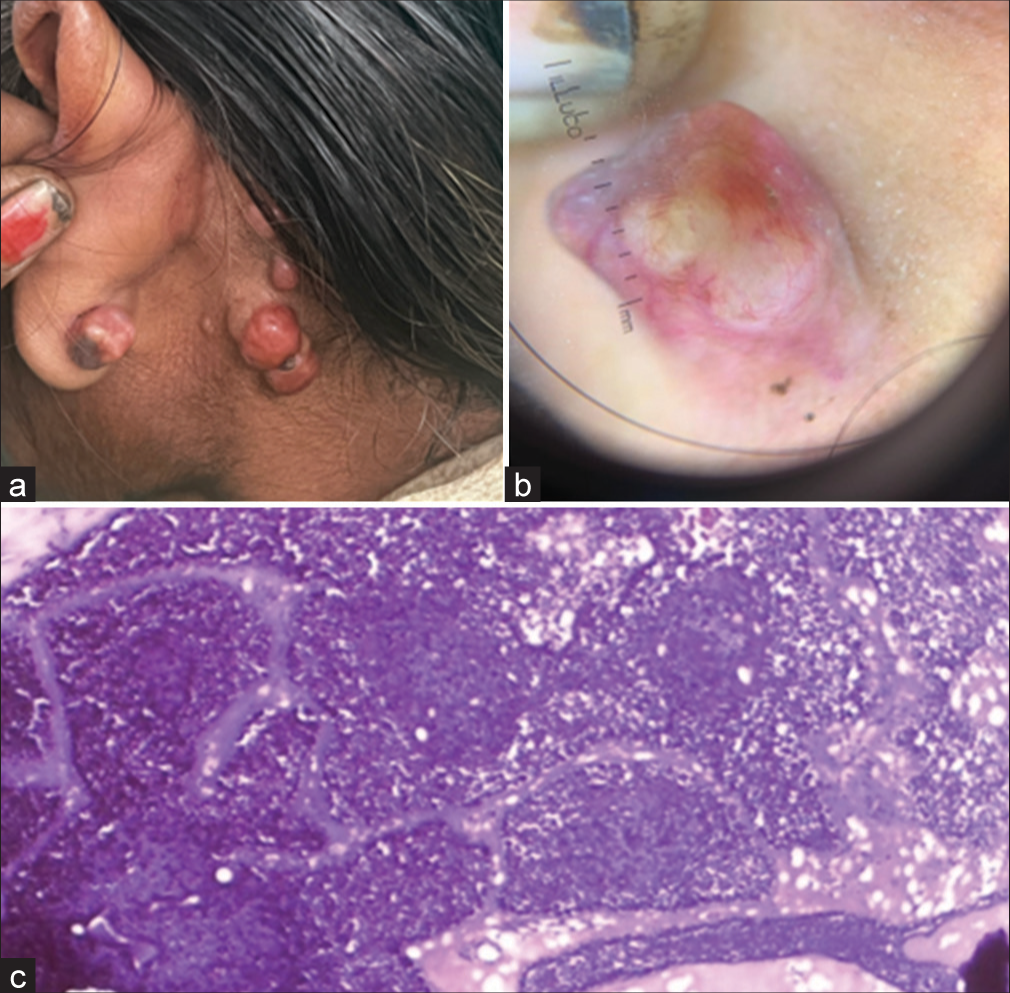
- (a) Clinical photograph of cylindroma showing multiple pink to red nodules, resembling a bunch of grapes. (b) Dermoscopy of cylindroma shows multiple arborizing telangiectasia branching toward periphery over a pink salmon background. (c) Histopathology shows peripheral cells exhibiting dark nuclei, whereas the central cells have a large, pale nuclei surrounding the lumina. (Magnification ×100).
In addition, there were a few nodules, ranging from 2 to 5 mm in size, with flat surfaces around the perioral area [Figure 2a]. Dermoscopy showed hyperpigmented dots and circles against a background of erythema and several structureless white areas [Figure 2b]. Histopathology of these nodules revealed multiple tumour islands with keratin cysts in a cribriform pattern [Figure 2c]. In addition, a few papulonodular lesions with a smooth surface, ranging from 3 to 5 cm in size, were present on the forehead [Figure 3a]. Dermoscopy revealed multiple branched vessels accompanied by bluish-black clods [Figure 3b]. Histopathology revealed multiple deeply basophilic lobules with collagenous cores, appearing as hazy areas within the cords [Figure 3c]. The patient had no other complaints aside from cosmetic concerns and discomfort, and the lesions were symptomless. No other family members had similar lesions.
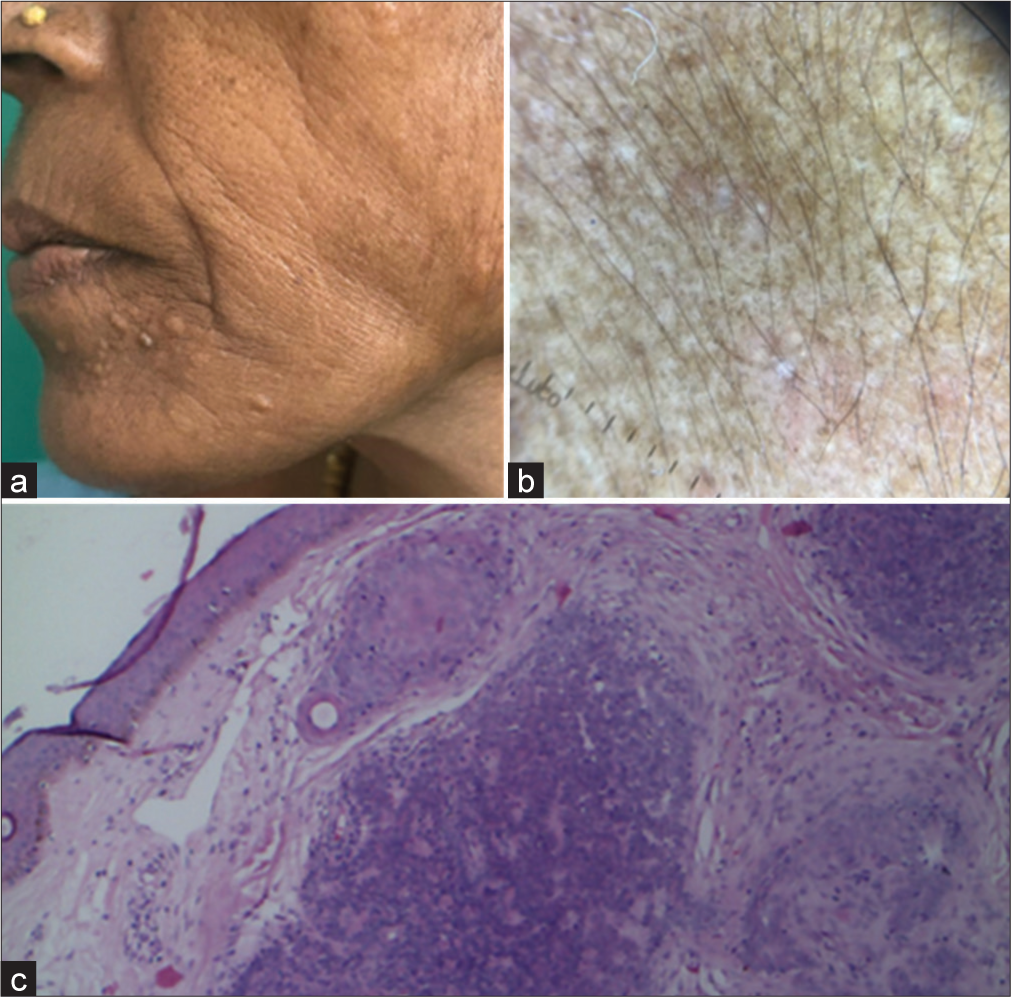
- (a) Trichoepithelioma – Clinical photograph shows dome-shaped to flat skin-coloured papules. (b) Dermoscopy showing hyperpigmented dots and circles with background erythema and multiple structureless white areas. (c) Histopathology showing tumor islands sharing cribriform pattern with germ and papilla structures (Magnification ×100).
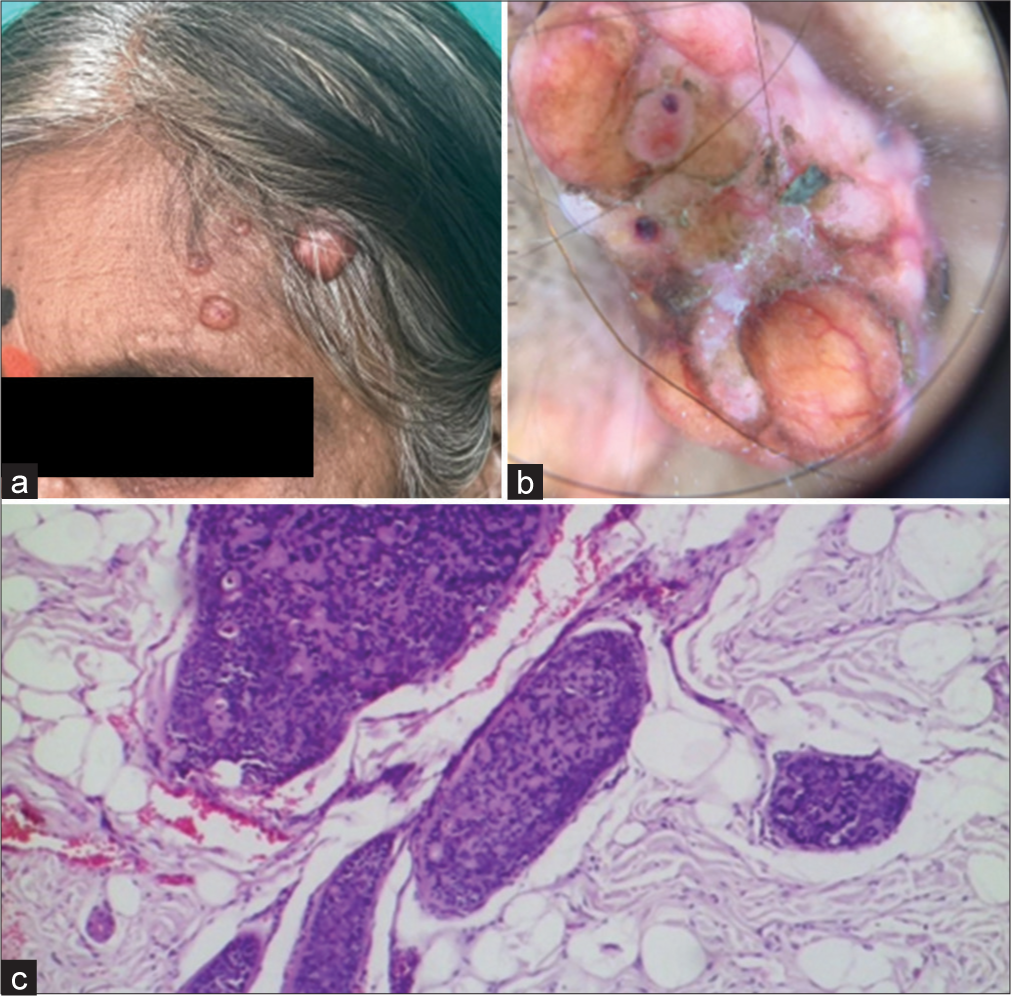
- (a) Spiradenoma – clinical photograph showing multiple skin-coloured nodules with a smooth surface. (b) Dermoscopy – multiple branched vessels with bluish-black clods. (c) Histopathology – multiple deeply basophilic lobules with collagenous core appearing as hazy areas seen within cords (Magnification ×100).
Exome sequencing of the CYLD gene using peripheral blood revealed no mutations. Patients with mosaic cylindroma carcinoma syndrome (CCS) should have tailored genetic testing based on family history. Those with postzygotic mutations typically lack a family history and may test negative for CYLD mutations in blood samples, unless leukocyte mosaicism exceeds 40%. A negative result should lead to DNA analysis from at least two skin tumour samples, preferably non-paraffin embedded tissue. Detecting a recurrent pathogenic CYLD variant in multiple skin tumours indicates mosaicism, aiding genetic testing of children or preimplantation genetic diagnosis.[4] Further, chromosomal assays may be needed to exclude other abnormalities. This case was diagnosed as sporadic, acquired, non-familial BSS in the HIV-positive patient based on clinical and histological features.
The patient was treated with serial excision of maximum possible lesions.
DISCUSSION
BSS is a rare autosomal dominant condition, typically manifesting in adolescence or early adulthood but can appear later. It predisposes individuals to multiple benign skin tumours, including cylindromas, trichoepitheliomas, and spiradenomas, primarily on the face, scalp, neck and occasionally the trunk. First reported by Ancell in 1842,[5] these tumours originate from sweat glands and hair follicles due to mutations in the CYLD gene, which normally acts as a tumour suppressor by regulating cell division and growth.
Beyond cutaneous manifestations, BSS can rarely involve the major and minor salivary glands and, very rarely, breast tumours.[6] Incidence is unknown. Phenotypic variants include multiple familial trichoepitheliomas, presenting without cylindromas or spiradenomas[7] and malignant cylindroma development in diagnosed BSS cases. Diagnosis involves clinical examination, family history, histopathology and CYLD gene mutation testing.[8]
This particular patient was a sporadic case of BSS with none of her family members affected, the patient was also HIV-positive. HIV infection primarily impacts the immune system and can cause various symptoms, opportunistic infections and certain cancers. While HIV infection can affect the skin, there is currently no evidence suggesting that it increases the risk of developing BSS, and neither has any other comorbidities such as diabetes and hypertension. However, there has been one study where silencing the ubiquitin-specific protease domain of the CYLD gene resulted in enhanced HIV transcription.[9] BSS is usually associated with the mutation of the CYLD gene, although there have been 2 reported cases of BSS that were not associated with CYLD gene mutation.[10] Patients with BSS should be closely monitored for recurrence and the development of malignant tumours. Although most cylindromas are benign, some may have malignant transformation and become potentially lethal. Malignant transformation of BSS tumours typically leads to visceral metastasis, resulting in a poor prognosis. Increased awareness is crucial for early diagnosis and effective management. Treatment options include excision, dermabrasion, electrodissection, carbon dioxide laser ablation, cryotherapy and radiotherapy. Inhibitors of nuclear factor-kappa B activity, such as sodium salicylate and prostaglandin A1, may help restore normal cell growth.[11]
CONCLUSION
BSS is a rare autosomal dominant disorder marked by benign skin tumours from sweat glands and hair follicles due to CYLD gene mutations. It typically appears in adolescence or early adulthood, affecting the face, scalp, neck and trunk, and occasionally the salivary glands and breasts. Diagnosis involves clinical examination, family history, histopathology and genetic testing. Awareness of phenotypic variants and potential malignancy is crucial for effective management.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Brooke-Spiegler Syndrome: A Rare Entity. Int J Trichology. 2012;4:29-31.
- [CrossRef] [Google Scholar]
- Brooke-Spiegler Syndrome and Phenotypic Variants: An Update. Head Neck Pathol. 2016;10:125-30.
- [CrossRef] [Google Scholar]
- Phenotypegenotype Correlations for Clinical Variants Caused by CYLD Mutations. Eur J Med Genet. 2015;58:271-8.
- [CrossRef] [Google Scholar]
- Diverse Presentations of Cutaneous Mosaicism Occur in CYLD Cutaneous Syndrome and May Result in Parent-to-child Transmission. J Am Acad Dermatol. 2019;81:1300-7.
- [CrossRef] [Google Scholar]
- History of a Remarkable Case of Tumours, Developed on the Head and Face; Accompanied with a Similar Disease in the Abdomen. Med Chir Trans. 1842;25:227-306.11.
- [CrossRef] [Google Scholar]
- Brooke-Spiegler Syndrome with Multiple Scalp Cylindromas and Bilateral Parotid Gland Adenomas. Case Rep Radiol. 2012;2012:249583.
- [CrossRef] [Google Scholar]
- Brooke-Spiegler Syndrome Variant Segregation of Tumor Types with Mixed Differentiation in Two Generations. Am J Dermatopathol. 1998;20:56-60.
- [CrossRef] [Google Scholar]
- Loss of the Cylindromatosis Tumour Suppressor Inhibits Apoptosis by Activating NF-κB. Nature. 2003;424:797-801.
- [CrossRef] [Google Scholar]
- Brooke-Spiegler Syndrome: Report of Two Cases not Associated with a Mutation in the CYLD and PTCH Tumor-suppressor Genes. J Cutan Pathol. 2012;39:366-71.
- [CrossRef] [Google Scholar]
- Familial Cylindromatosis and Brooke-Spiegler Syndrome. Dermatol Surg. 2009;35:845-52.
- [CrossRef] [Google Scholar]







