Translate this page into:
Efficacy and Safety of Crisaborole 2% Ointment in the Treatment of Mild-to-moderate Atopic Dermatitis – A Prospective, Open-label Study at a Tertiary Care Centre in Eastern India
*Corresponding author: Shini Choubey, Department of Dermatology, Kalinga Institute of Medical Sciences, Bhubaneshwar, Odisha, India. choubeyshini@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Begum F, Behera D, Raj C, Choubey S. Efficacy and Safety of Crisaborole 2% Ointment in the Treatment of Mild-to-moderate Atopic Dermatitis – A Prospective, Open-label Study at a Tertiary Care Centre in Eastern India. Indian J Postgrad Dermatol. 2025;3:7-12. doi: 10.25259/IJPGD_170_2024
Abstract
Objectives:
Atopic dermatitis (AD) is a common occurrence worldwide, where topical corticosteroids and calcineurin inhibitors, along with antihistamines, are being used for the treatment of mild-to-moderate AD. Crisaborole 2% ointment, a phosphodiesterase-4 inhibitor, has been recently approved for AD treatment by the Food and Drug Administration. Crisaborole is proposed to have lesser side effects in comparison to its counterparts. However, the impact of crisaborole on the Indian population has only been studied in the paediatric population and has not been widely explored. We conducted this study to find the safety and efficacy of crisaborole 2% ointment in mild-to-moderate AD in the Indian population.
Materials and Methods:
Thirty consecutive mild-to-moderate AD patients were included in the study. The patients were advised to apply crisaborole 2% ointment at the site of involvement twice daily for 28 days. The evaluation was done at baseline and day 28 using the SCORing AD (SCORAD), investigator static global assessment (ISGA) and eczema-associated severity index (EASI) scores. The patients were followed up to report any side effects.
Results:
There was a statistically significant decrease in the mean SCORAD, ISGA and EASI scores after the end of treatment. According to SCORAD, there were 58.3% of patients with moderate AD which reduced to 41.6% at the end of treatment. ISGA defined treatment success as only being achieved by 36.6% of patients, out of which complete resolution was achieved only by 20% of patients at the end of treatment. The mean EASI scores showed a considerable decreasing trend from 8.47 at baseline to 4.73 at the end of the study. Six patients withdrew from the study due to exacerbation of symptoms, possibly burning at the site of application.
Conclusion:
Although crisaborole reduced symptoms, it did not provide any instant relief to symptoms of AD, leading to discontinuation in a few patients, making it a viable drug for maintenance rather than a first-line therapy.
Keywords
Atopic dermatitis
PDE4 inhibitor
Topical crisaborole
INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin disorder that is estimated to affect 15–30% of children and 2–10% of adults in developed countries.[1] Disease manifestations and distribution vary with age. It can manifest as red, eczematous lesions, crusted erosions or lichenified lesions associated with severe pruritus. The breakdown of the epidermal barrier, genetic predisposition and immune system dysfunction play a complex interaction in the genesis of AD. The majority of patients with AD have mild-to-moderate disease and are treated with various topical medications such as topical steroids and calcineurin inhibitors. Systemic modalities such as steroids, methotrexate and cyclosporine are advised for patients with moderate-to-severe disease or disease involving large surface areas. Dupilumab and tralokinumab are Food and Drug Administration (FDA)- approved biologics for recalcitrant and resistant diseases.[2]
A novel approach to treating AD involves phosphodiesterase 4 (PDE4) inhibitors, which have been shown to inhibit pro-inflammatory responses involved in AD pathogenesis, including cytokine release. Treatment with PDE4 inhibitors is best suited as topical therapy to limit systemic drug exposure and adverse effects. In recent years, a family of boron-based compounds known as phenoxy benzoxaboroles has been shown to inhibit PDE4 at micromolar concentrations effectively. Crisaborole topical ointment, 2% (previously known as AN2728 topical ointment, 2%; Anacor Pharmaceuticals, Inc., Palo Alto, CA) is a novel, boron-based, topically administered PDE4 inhibitor that has demonstrated anti-inflammatory properties in biochemical, cell-based and animal studies.[3] It does not have a black box warning, and studies have confirmed that pruritus is reduced over 2–3 days after application.[4] To the best of our knowledge, only one research has been done on the effectiveness of crisaborole 2% ointment in treating AD in the Indian pediatric population, but none have been done on the treatment of AD in the adult Indian community. The study aimed to assess the efficacy and side effect profile of crisaborole 2% ointment for the treatment of AD in the Indian population.
MATERIALS AND METHODS
This prospective study was conducted in the outpatient department (OPD) of dermatology of a tertiary care centre in eastern India from November 2023 to April 2024. The approval from Institutional ethics committee was acquired.
The sample size was calculated by convenience sampling. Thirty consecutive patients satisfying the inclusion criteria were included in the study. A written informed consent was obtained.
Inclusion criteria
Patients clinically diagnosed with mild-to-moderate AD according to Hanifin and Rajka criteria with a maximum SCORAD of 50
Patients above 2 years of age.
Exclusion criteria
Pregnant or lactating females
Extensive (>10%) body surface area involvement
Patients with any active skin infections
Severe systemic illness (uncontrolled diabetes mellitus or hypertension, liver or renal diseases)
Immunocompromised patients
Patients who were previously treated with topical or systemic corticosteroids/biologics.
The subjects were advised to apply crisaborole 2% ointment twice daily over the affected area for 28 days. The assessment was done at the initial visit and on day 28 of treatment. The severity of the disease was assessed using SCORing AD (SCORAD) with mild (<25), moderate (25–50) and severe (>51). The severity was recorded at the initial visit (day 0) and on day 28 after the completion of the treatment. Investigator static global assessment score (ISGA) was calculated and compared on day 28 from the baseline, and the primary efficacy end point of success in ISGA was defined as clear (0) or almost clear (1) with a 2-grade or more improvement from baseline. Eczema-associated severity index (EASI) was noted along with the clinical photographs. The clinical photographs and percentage improvement using EASI were noted [Figure 1]. The patients were followed up and were asked to report to the outpatient department following any treatment-related adverse effects. Crisaborole was discontinued in these patients, and mid-potent topical corticosteroids were prescribed for 15 days.
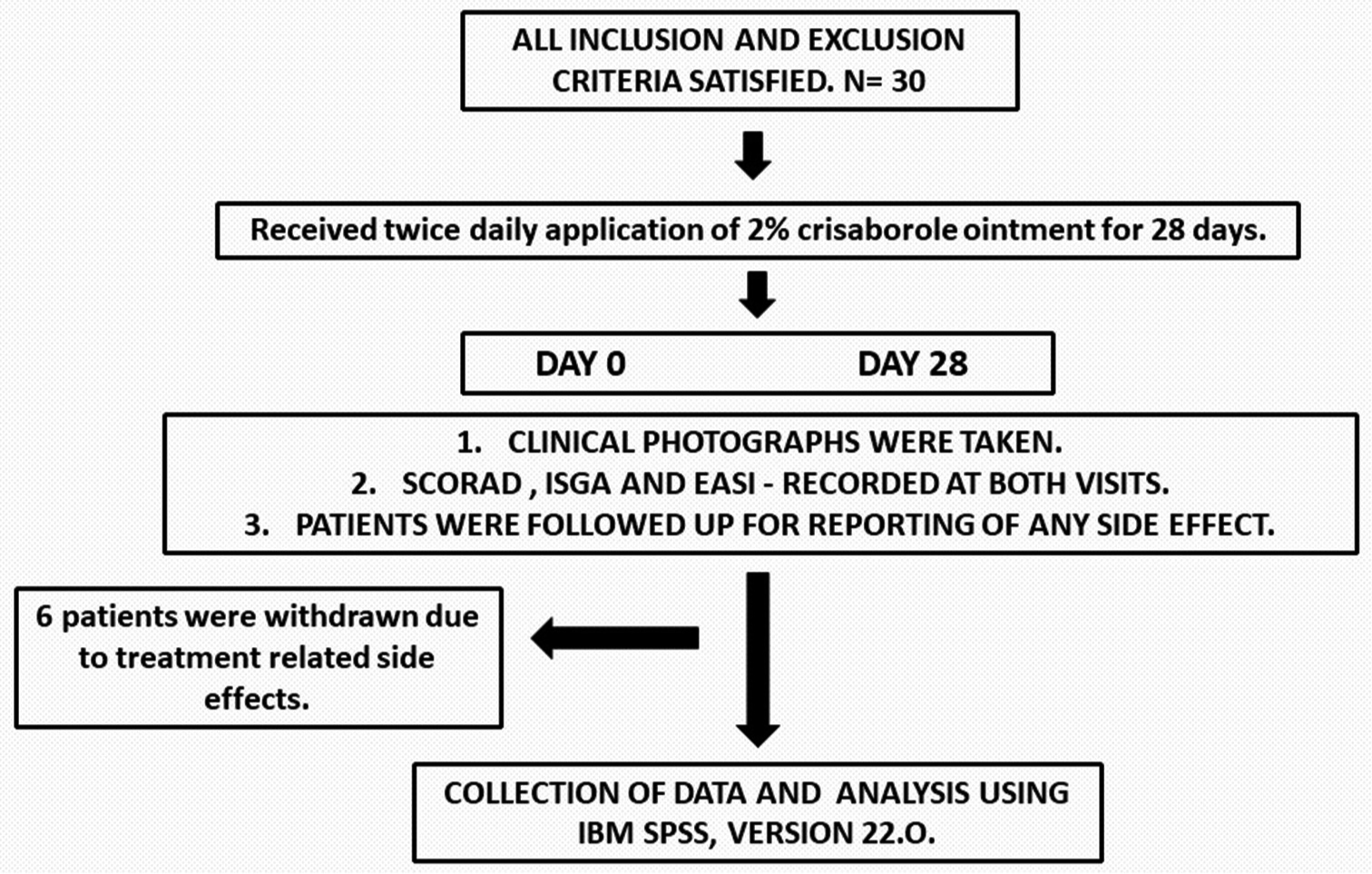
- Study’s flowchart. SCORAD: SCORing for Atopic Dermatitis, ISGA: Investigator static global assessment, EASI: Eczema associated severity index.
Statistical analysis
All the continuous variables were presented in the mean ± standard deviation, and categorical variables were in frequency and percentage. Initial and final value comparison was done using a paired t-test. P < 0.05 was considered as statistically significant. Statistical analysis was done using IBM - Statistical Package for the Social Sciences V26.
RESULTS
A total of 30 patients were included in our study. The male-to-female ratio was 1:9. The mean age of patients was 31 years ± 15.7. The demographical details of all the participants are mentioned in Table 1. The most common affected site was the hands in this study (23.3%). Six patients were excluded from the analysis due to reporting of side effects. The mean SCORAD at baseline was 29.47, and the final mean SCORAD was 20.64, which was statistically significant with a P < 0.001. The severity of the disease was assessed by SCORAD, and at the baseline, there were 10 (41%) patients with mild AD (score <25), while 14 (59%) patients had moderate (Score 25–50) AD. On the day of assessment, 14 (59%) patients had mild AD, and 10 (41%) had moderate AD. Similarly, both mean ISGA and mean EASI scores showed considerable improvement by the end of the study, i.e., from 3.17 at baseline to 2.04 at day 28 and 8.47 at baseline to 4.73 by the end of the study, respectively. These results were statistically significant, with a P < 0.001 [Table 2]. Complete resolution of symptoms was achieved by six patients (20%) at the end of treatment [Table 3].
| Age (Mean±SD) | 31±15.7 |
| Sex, n (%) | |
| Female | 21 (70.0) |
| Male | 9 (30.0) |
| Site, n (%) | |
| Face | 4 (13.3) |
| Feet | 4 (13.3) |
| Finger tip | 5 (16.7) |
| Hand | 7 (23.3) |
| Neck | 2 (6.7) |
| Periorbital | 2 (6.7) |
| Toes | 6 (20) |
SD: Standard deviation
| SCORAD | |||
|---|---|---|---|
| Baseline | Day 28 | P-value | |
| A. Mean score | 29.47±11.83 | 20.64±16.22 | <0.005 |
| B. Severity Score | |||
| Mild (<25) | 10 | 14 | |
| Moderate (25–≤50) | 14 | 10 | |
| Severe (>51) | 0 | 0 | |
SCORAD: SCORing Atopic Dermatitis
| Baseline | Day 28 | P-value | |
|---|---|---|---|
| Mean | Mean | ||
| A. Mean score | 3.17±0.717 | 2.04±1.296 | <0.001 |
| B. ISGA results based on site | |||
| Site | Failure | Success | P-value |
| Face | |||
| n | 0 | 4 | 0.015 |
| % | 0.00 | 36.40 | |
| Feet | |||
| n | 4 | 0 | |
| % | 30.70 | 0.00 | |
| Finger tip | |||
| n | 4 | 1 | |
| % | 30.70 | 9.10 | |
| Hand | |||
| n | 0 | 2 | |
| % | 0.00 | 18.20 | |
| Neck | |||
| n | 0 | 2 | |
| % | 0.00 | 18.20 | |
| Periorbital | |||
| n | 2 | 0 | |
| % | 15.30 | 0.00 | |
| Toes | |||
| n | 3 | 2 | |
| % | 23.00 | 18.20 | |
| C. ISGA results | |||
| n | % | ||
| Discontinued | 6 | 20 | |
| Failure | 13 | 43.3 | |
| Success | 11 | 36.6 | |
| Total | 30 | 100 | |
ISGA: Investigator static global assessment
Based on the site of involvement, the lesions distributed over the face, hand and neck had a better response with a 100% success rate [Figures 2 and 3]. On the contrary, the periorbital area, fingertip, feet and toes were challenging to treat and did not respond well [Figure 4].
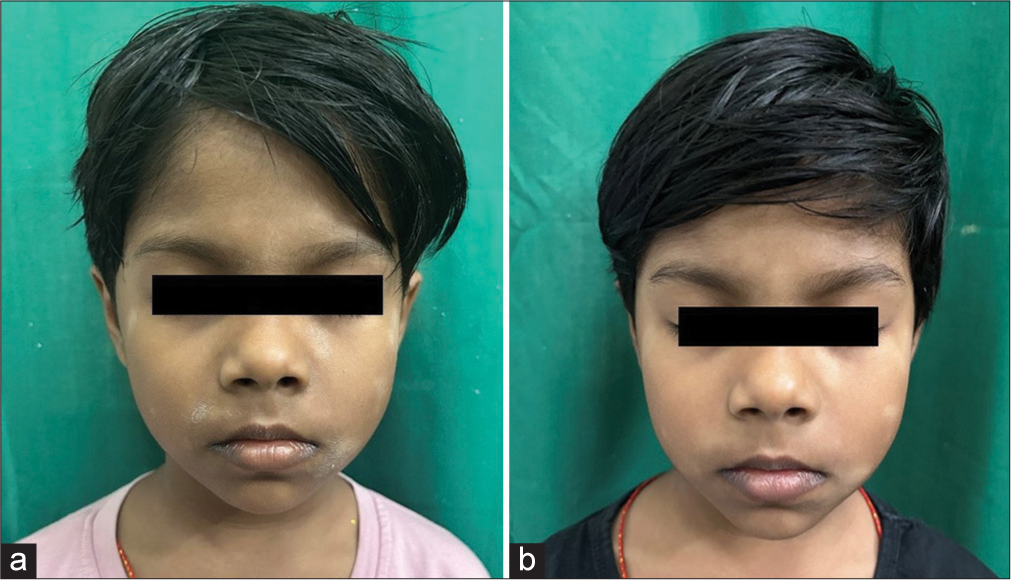
- (a) Multiple well defined hypopigmented macules of varying size with mild scaling present over the face. (b) Decrease in the number of macules and complete resolution of scaling after twice daily application of crisaborole 2% ointment for 28 days.
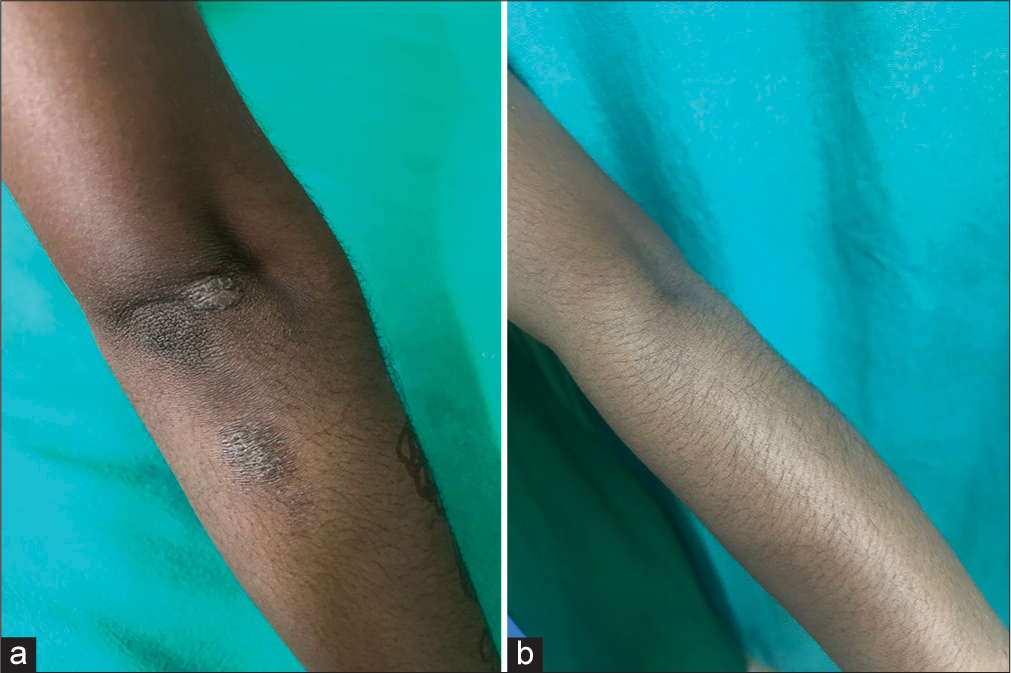
- (a) A well defined lichenified plaque measuring 1 × 2 cm over the extensor aspect of the left hand. (b) Complete resolution of lichenification and scaling over the lesion after twice daily application of crisaborole 2% ointment for 28 days.
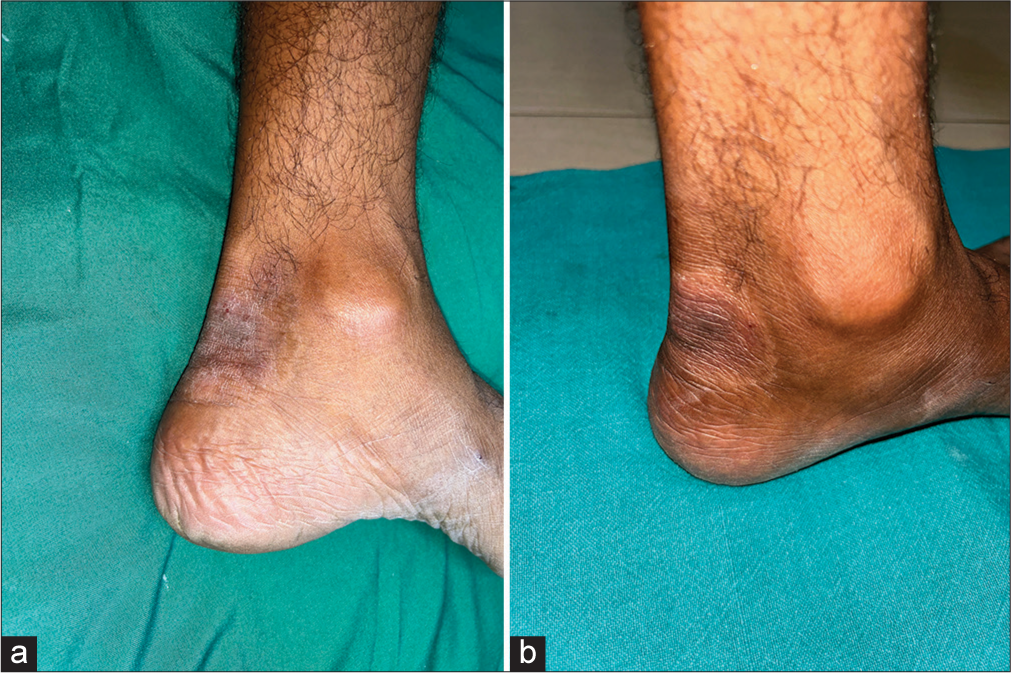
- (a) A well-defined, oval, erythematous to hyperpigmented plaque present over the posterior aspect of the right foot with scaling. (b) Mild improvement was seen in the form of a decrease in erythema and scaling of the plaque after twice daily application of crisaborole 2% ointment for 28 days.
Out of 30, six patients discontinued the application of the drug due to the treatment-related adverse effects, such as acute flare of the pre-existing lesion, which was seen in two patients (6.6%), and four patients experienced pain and burning sensation at the site of application within 4–7 days of starting the treatment (13.2%) [Table 4]. Out of the 24 patients who continued to participate in the study, only 11 patients (36.6%) could achieve treatment success, according to ISGA, by the end of 28 days. All the patients who discontinued crisaborole were prescribed mid-potent topical corticosteroids for 15 days.
| S. No. | Age | Sex | Site | Baseline | ||
|---|---|---|---|---|---|---|
| ISGA | EASI | SCORAD | ||||
| 1. | 66 | F | Hand | 4 | 12 | 34.5 |
| 2. | 30 | F | Toes | 2 | 7 | 24.05 |
| 3. | 55 | F | Finger tip | 3 | 8 | 29 |
| 4. | 25 | M | Hand | 3 | 10 | 42.05 |
| 5. | 70 | F | Feet | 4 | 12 | 69 |
| 6. | 33 | F | Toes | 2 | 7 | 34.5 |
ISGA: Investigator static global assessment, EASI: Eczema associated severity index, SCORAD: SCORing Atopic Dermatitis
DISCUSSION
AD is one of the most common, chronic and recurring diseases seen in any dermatology OPD. Patients are required to take treatment for a long time to keep the disease in remission and to prevent flare. Until very recently, topical corticosteroids and calcineurin inhibitors were being used as the topical treatment for AD. However, recently, crisaborole 2% ointment, a novel boron-based PDE4 inhibitor, was approved by the FDA in December 2016 for patients of AD for above 2 years of age and in March 2020 for patients above 3 months respectively as a topical non-steroidal medication that has demonstrated anti-inflammatory properties and has been in use ever since in the western population for the treatment of mild-to-moderate AD.[5] Patients have also reported improvement in pruritus earlier than those treated with vehicle.[4]
Crisaborole, when applied topically, penetrates the epidermis and dermis and reaches quantifiable levels in the plasma. The protein binding is 97% predominantly to albumin. The metabolism is through its several inactive metabolites through hydrolysis and oxidation, eliminated through the kidneys. Its advantage over topical corticosteroids and calcineurin inhibitors is the eliminated risk of development of malignancies in the future, making it a safer drug for long-term management. The drug is presumed to be safe in the elderly population, but there is scanty data to assess its safety in pregnancy or lactation.[6,7]
The study included patients aged 2+ with mild-to-moderate AD with <10% body surface area (BSA) involvement. SCORAD which was used as a parameter to assess the disease severity, showed a decrease in 18% of patients post-treatment. Treatment with crisaborole reduced mean SCORAD from 29.47 to 20.64, and 40% of patients achieved ISGA score success. The percentage of patients achieving clear or almost clear scores, according to ISGA, was 30.3%. However, the severity of clinical signs and symptoms on day 28 showed a statistically significant improvement in ISGA (P = 0.001) and a decrease in the body surface area involvement as compared to the baseline, which is similar to the finding of Silverberg et al.[8,9] Since the aetiology of AD is multifactorial, disruption to the skin barrier and subsequent superadded infection by staphylococcus play an important role in the pathogenesis of the disease. Ingredients included in vehicles are likely to boost the skin’s innate immune defences and induce additional changes in the skin, like skin barrier restoration.[10]
In this study, 16% of patients showed intolerance to the preparation and discontinued the application of preparation. Most treatment-related adverse effects were mild burning and pain at the application site. In our study, six patients discontinued the application – two due to flaring of the lesion and discomfort and four due to pain or burning at the site of application. However, studies in the Western population have shown a good safety profile and minimal side effects.[11]
An Indian study by De et al. studied the effect of crisaborole ointment in 19 pediatric patients with AD. They found that the mean ISGA score had declined from 2.58 ± 0.61 to 0.95 ± 0.78 with a reduction in signs and symptoms of AD. Localized burning at the site of application was seen as a side effect in a few patients which was similar to our study.[12]
Prior research has indicated an increased degree of intolerance to specific skin preparations amongst Asian patients as compared to White patients. Thus, the increased application site pain due to crisaborole in Chinese and Japanese patients may have been partially explained by the study population’s exclusivity of Asian patients, supporting a similar finding in our study of the Indian population.[13,14] Due to a thinner stratum corneum or a more significant density of sweat glands, Asian individuals have been seen to be more sensitive to chemical stimuli on their skin.[14,15] This could explain the increased frequency of observed cutaneous side effects. Notably, application site discomfort caused by crisaborole has been reported to be transient and self-resolving.[15] This can be minimised by storing it in the refrigerator before use. No other new treatment-related adverse effect was recorded.
According to a study by Eichenfeild et al., burning or stinging at the site of application of crisaborole ointment was recorded in 4% of the studied population. This is comparable to or lower than that of other topical therapies for mild-to-moderate AD. Application site burning was reported as an adverse effect of other topical agents used to treat AD; 1-6% of patients treated with corticosteroids, 20-58% with tacrolimus, and 8-26% with pimecrolimus respectively.[16]
Patients with facial, neck and hand involvement had a better response than the periorbital region, feet and toes. Further, anatomical site-based studies can help us assess the best potential use of the drug. A study by Zane et al. showed that crisaborole was well tolerated in sensitive areas such as intertriginous areas, genitals and face/hairline.[17] Further, prospective studies are warranted to establish this. It can help us decide the course of management for the patient based on the site of involvement.
Limitation
A limited sample size.
As the duration of the study was short it was not possible to observe long-term adverse effects.
CONCLUSION
Previous studies have mostly assessed the Western population; therefore, it is critical to evaluate the safety and efficacy of crisaborole in the Indian population. However, in our experience, there was no instant relief of mild AD symptoms, and patients reported flare of the disease, pain and burning sensation at the site of application, which led to the medication being discontinued in a few patients, making it a viable drug for maintenance therapy that can be continued for an extended period rather than a first-line drug.
Ethical approval
The study approved by Institutional Review Board at Kalinga Institute of Medical Sciences, Bhubaneshwar, Odisha, number KIMS/SLRC/82/2023, dated 13th April 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Atopic Dermatitis In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2024.
- [Google Scholar]
- A Phase 2, Randomized, Controlled, Dose-ranging Study Evaluating Crisaborole Topical Ointment, 0.5% and 2% in Adolescents with Mild to Moderate Atopic Dermatitis. J Drugs Dermatol. 2015;14:1394-9.
- [Google Scholar]
- Crisaborole 2% Ointment (Eucrisa) for Atopic Dermatitis. Skin Therapy Lett. 2019;24:4-6.
- [Google Scholar]
- Safety, Effectiveness, and Pharmacokinetics of Crisaborole in Infants Aged 3 to < 24 Months with Mild-to-Moderate Atopic Dermatitis: A Phase IV Open-Label Study (CrisADe CARE 1) Am J Clin Dermatol. 2020;21:275-84.
- [CrossRef] [PubMed] [Google Scholar]
- Crisaborole 2% Ointment for Mild-to-Moderate Atopic Dermatitis. Skin Therapy Lett. 2021;26:1-4.
- [Google Scholar]
- Crisaborole Ointment, 2%, for Treatment of Patients with Mild-to-moderate Atopic Dermatitis: Systematic Literature Review and Network Meta-analysis. Dermatol Ther (Heidelb). 2020;10:681-94.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and Safety of Crisaborole Ointment, A Novel, Nonsteroidal Phosphodiesterase 4 (PDE4) Inhibitor for the Topical Treatment of Atopic Dermatitis (AD) in Children and Adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluating the Efficacy of Crisaborole Using the Atopic Dermatitis Severity Index and Percentage of Affected Body Surface Area. Acta Derm Venereol. 2020;100:5744.
- [CrossRef] [PubMed] [Google Scholar]
- Current and Emerging Therapies for Atopic Dermatitis in the Elderly. Clin Interv Aging. 2023;18:1641-52.
- [CrossRef] [PubMed] [Google Scholar]
- Vehicles for Atopic Dermatitis Therapies: More than Just a Placebo. J Dermatolog Treat. 2022;3:685-98.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and Safety of Crisaborole Ointment, 2%, for the Treatment of Mild-to-Moderate Atopic Dermatitis Across Racial and Ethnic Groups. Am J Clin Dermatol. 2019;20:711-23.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and Safety of Crisaborole Ointment in Pediatric Atopic Dermatitis: A 4-Week Open-label Study. Indian J Skin Allergy. 2024;3:60-5.
- [CrossRef] [Google Scholar]
- Efficacy and Safety of Crisaborole Ointment in Chinese and Japanese Patients Aged =2 Years with Mild-to-Moderate Atopic Dermatitis. J Dermatol. 2023;50:847-55.
- [CrossRef] [PubMed] [Google Scholar]
- Racial Differences in Acute and Cumulative Skin Irritation Responses between Caucasian and Asian Populations. Contact Dermatitis. 2000;42:134-43.
- [CrossRef] [PubMed] [Google Scholar]
- Peak Pruritus Numerical Rating Scale: Psychometric Validation and Responder Definition for Assessing itch in Moderate-to-severe Atopic Dermatitis. Br J Dermatol. 2019;181:761-9.
- [CrossRef] [PubMed] [Google Scholar]
- Once-Daily Crisaborole Ointment, 2%, as a Long-Term Maintenance Treatment in Patients Aged = 3 Months with Mild-to-Moderate Atopic Dermatitis: A 52-Week Clinical Study. Am J Clin Dermatol. 2023;24:623-35.
- [CrossRef] [PubMed] [Google Scholar]
- Tolerability of Crisaborole Ointment for Application on Sensitive Skin Areas: A Randomized, Double-Blind, Vehicle-Controlled Study in Healthy Volunteers. Am J Clin Dermatol. 2016;17:519-26.
- [CrossRef] [PubMed] [Google Scholar]







