Translate this page into:
Acute Methotrexate Toxicity in a Case of Generalised Lichen Planus
*Corresponding author: Arun C. Inamadar, Department of Dermatology, Venereology and Leprosy, Shri B M Patil Medical College, Hospital and Research Centre, BLDE (Deemed to be) University, Vijayapura, Karnataka, India. aruninamadar@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Marri SS, Bilkhiwal E, Janagond AB, Inamadar AC. Acute Methotrexate Toxicity in a Case of Generalised Lichen Planus. Indian J Postgrad Dermatol. doi: 10.25259/IJPGD_18_2025
Dear Editor,
Methotrexate (MTX) toxicity occurring in cases of psoriasis and rheumatoid arthritis has been widely reported. Here, we report a case of generalised lichen planus (LP), who presented with ulceration of skin lesions, mucosal ulceration and haematological derangements after treatment with MTX. This report is unprecedented as MTX toxicity in LP has been hitherto unreported to the best of our knowledge.
A 60-year-old male presented with itchy skin lesions all over the body for 6 months. The patient had uncontrolled diabetes mellitus (haemoglobin A1C - 10.3%) and was on oral metformin for the past 12 years. On examination, multiple well-defined violaceous flat-topped scaly papules and plaques were present over the trunk and upper extremities and lichenified plaques over the lower extremities involving a body surface area of 30–40%. There were no lesions in oral cavity. Nail examination revealed multiple longitudinal ridges over all fingernails and pincer nail deformity with subungual hyperkeratosis of both great toenails. Skin biopsy for histopathological examination was advised, but the patient refused. Polarised dermoscopy using handyscope (FotoFinder™ systems GmbH, Bavaria, Germany) showed violaceous background with linear white streaks and peripheral brown dots [Figure 1]. A diagnosis of LP was established based on the clinical and dermoscopic features. The patient was advised oral MTX 15 mg/week after necessary investigations.
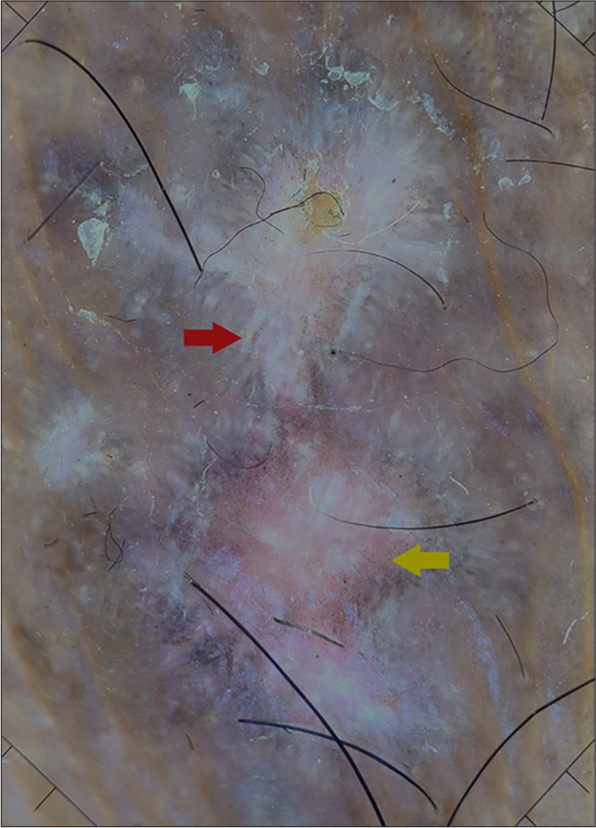
- Dermoscopy showing a violaceous background with linear white streaks (Wickham’s striae, red arrow) and peripheral brown dots (yellow arrow) (FotoFinder™ systems GmbH, Bavaria, Germany. Polarised ×20).
The patient developed ulceration and pain over skin lesions and mucosal erosions 2 days after the second MTX dose. On examination, multiple erosions and crusting over few existing skin lesions were present. Few erosions were present over buccal mucosa [Figure 2a and b].
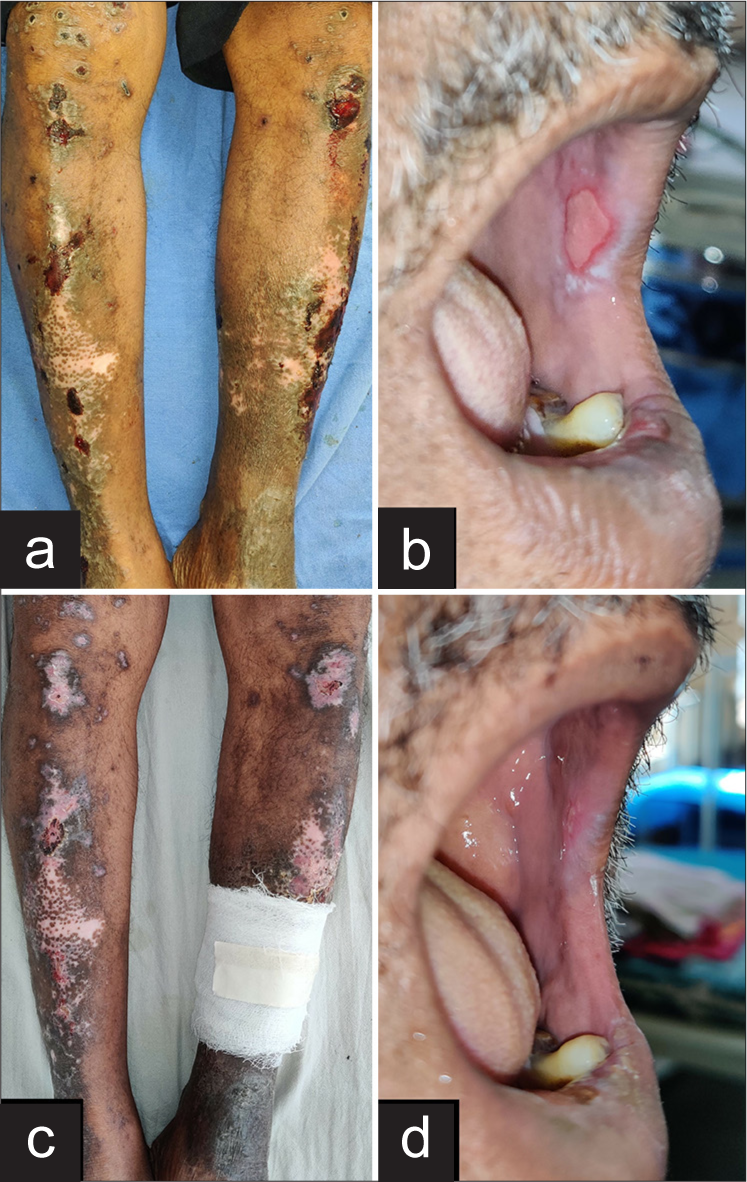
- Clinical images showing (a) ulceration over lichen planus lesions over legs and (b) erosions in oral cavity at presentation (c) resolution of cutaneous ulceration and (d) resolution of erosions in oral cavity after 10 days of treatment initiation.
On evaluation, the patient was found to have leucopenia, thrombocytopenia, albuminuria and glycosuria [Table 1]. Liver and renal function tests were normal. Serum MTX levels could not be measured. A provisional diagnosis of acute MTX toxicity was made, and the patient was started on intravenous leucovorin 20 mg every 6 h, along with vigorous hydration under intensive care. Prophylactic antibiotics and a single subcutaneous dose of pegfilgrastim 6 mg were administered. Skin lesions were cleaned, and topical antibiotic dressing was done.
The patient experienced a full recovery with haematological abnormalities returning to normal after 2 days and resolution of mucocutaneous ulceration after 10 days of treatment [Table 1, Figure 2c and d]. Systemic retinoid was started for the management of LP.
| At baseline | After 48 h of administering pegfilgrastim | After 10 days of administering pegfilgrastim | |
|---|---|---|---|
| TLC | 1490/µL | 4980/µL | 13960/µL |
| Neutrophils | 56.1% | 48.2% | 75.0% |
| Platelets | 54000/µL | 82000/µL | 547000/µL |
TLC: Total leucocyte count
LP is an immunological disorder involving skin, mucosa, hair and nails. Treatment of LP is directed towards the resolution of symptoms and is essential for preventing complications such as scarring and rarely carcinoma. According to the European guidelines, corticosteroids are the first-line treatment, which is also most commonly employed by treating physicians. MTX at a dose of 15– 20 mg/week is the third-line treatment for cutaneous LP.[1] MTX acts on dihydrofolate reductase, an enzyme required for the synthesis of purines and pyrimidines and inhibits it, thereby hindering the proliferation of activated T-cells and keratinocytes. The use of this drug for inflammatory disorders is a common practice due to its low cost and high efficacy. Multiple studies conducted on LP patients have shown that MTX is safe.[2]
MTX toxicity commonly occurs due to dosage errors either by the patient or physician. Other causes include renal impairment, hypoalbuminaemia, use of drugs interacting with MTX and genetic polymorphisms in folate pathway.[3,4] In the present case, there was no clear identifiable cause.
Prolonged intracellular presence of the drug is due to polyglutamination, seen in cells such as leukemic myeloblasts, synovial macrophages, lymphoblasts and epithelia. Acute toxicity causes dermatitis, mucositis, gastrointestinal bleeding, diarrhoea and myelosuppression. Other features include neutropenic fever, ischaemia, haemorrhage, electrolyte and fluid losses, hepatotoxicity and pulmonary toxicity. Precipitation of MTX and its metabolites in renal tubules occurs in acidic environment which leads to renal damage.[3] In the present case, the patient had developed mucocutaneous ulceration, leucopenia and thrombocytopenia. Ulceration of psoriatic plaques maybe due to higher uptake of MTX by hyperproliferative lesions.[5] Ulceration of LP lesions could be mediated by a similar mechanism.
Prompt recognition of symptoms and signs of MTX toxicity and admission to intensive care unit for further management is necessary to prevent complications. Treatment requires rapid hydration, alkalinisation of urine, haemodialysis (facilitates rapid elimination of drug), leucovorin and glucarpidase. Granulocyte colony-stimulating factor can be used for the management of neutropenia. Barrier dressings and oral care should be practiced.[3] This patient was successfully managed with vigorous hydration, leucovorin and a single dose of pegfilgrastim.
Although the safety profile of MTX in LP has been proven in multiple studies, risk of toxicity still persists, and the patient should be monitored carefully for early detection and prompt treatment.
Ethical approval:
Institutional Review Board approval is not required.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- European S1 Guidelines on the Management of Lichen Planus: A Cooperation of the European Dermatology Forum with the European Academy of Dermatology and Venereology. J Eur Acad Dermatol Venereol. 2020;34:1403-14.
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate for Treatment of Lichen Planus: Old Drug, New Indication. J Eur Acad Dermatol Venereol. 2013;27:e410-3.
- [CrossRef] [Google Scholar]
- Influence of Genetic Polymorphisms in the Folate Pathway on Toxicity After High-dose Methotrexate Treatment in Pediatric Osteosarcoma. Blood Res. 2016;51:50-7.
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate Toxicity During Treatment of Chronic Plaque Psoriasis: A Case Report and Review of the Literature. Dermatol Ther (Heidelb). 2014;4:145-56.
- [CrossRef] [PubMed] [Google Scholar]







