Translate this page into:
Boxed Warnings in Dermatotherapeutics
*Corresponding author: Yogesh S. Marfatia, Department of Skin and Venereology, SBKS Medical Institute and Research Centre, Sumandeep Vidyapeeth Deemed to be University, Vadodara, Gujarat, India. ym11256@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jinwala PP, Marfatia YS, Rout P, Khanna S, Talati A. Boxed Warnings in Dermatotherapeutics. Indian J Postgrad Dermatol. 2025;3:19-26. doi: 10.25259/IJPGD_2_2025
Abstract
Boxed warnings (BWs), more commonly known as ‘Black Box warnings,’ are safety-related warnings assigned to medications by the Food and Drug Administration of the United States of America. Adverse events of the most serious kind and new data that emerged through post-marketing surveillance are highlighted. Furthermore, they implore the physician to pay heed to other important matters such as dosing, monitoring protocols as well as probable drug interactions. In the prescribing information document for any drug, special emphasis is placed on the BW by highlighting it with a black border and placing it on the top of the list of adverse drug reactions (ADRs). Commonly prescribed systemic medications for which BWs have been issued include azathioprine, itraconazole, cyclosporine, ciprofloxacin, tofacitinib, oral retinoids and rituximab. BW related to topical calcineurin inhibitors generated a lot of debate. The issues and challenges related to BW include financial and marketing aspects, undue apprehension in the minds of physicians and patients, medicolegal aspect. The onus is on the physician to be aware of such a warning and to assess the risks versus benefits before prescribing such drugs. Participation of the physician in post-marketing surveillance is essential in knowing about previously unknown ADRs. Through this article, the idea that these BWs are not to be viewed as complete contraindications but as an important guiding tool that should not be ignored has been explored.
Keywords
Boxed warning
Janus kinase inhibitors
Omalizumab
Rituximab
Topical calcineurin inhibitors
USFDA
INTRODUCTION
The safety data of newly approved drugs are based on short-term clinical trials conducted in limited study populations. It may not be applicable to the general population at large. When the drug is used by a large number of patients and for a longer period, new data related to adverse events emerge, mostly through post-marketing surveillance. Of these newly reported adverse events, the most serious type of adverse events is highlighted as Boxed Warnings (BWs).
WHAT DOES “BOXED WARNING” MEAN?
The Food and Drug Administration which is the drug control authority of the United States of America (USFDA) issues BW (previously known as Black Box Warning) on medications.
The producer’s prescribing guidelines (often referred to as the package insert) has a black border around the BW, so that it is apparent and will be immediately seen by a prescriber, who can understand the gravity of the warning. Potential hazards are listed in descending order beneath the BW in the sections labelled ‘Adverse Reactions’, ‘Warnings and Precautions’ and ‘Contraindications’.
A BW applies not to one specific drug, but rather to the entire class that it belongs to, as usually the grave risk is related to the mechanism of action and its unwanted effects on the body. At present, over 400 medications have BW, while up to 20% drugs are likely to acquire new BW or be withdrawn from the market over the next 25 years.[1,2]
BWS ARE ISSUED FOR THE FOLLOWING THREE SITUATIONS
When an adverse reaction is serious enough (potentially permanently disabling or fatal reactions) compared to advantages of the drug, it is necessary to assess the risk versus advantage of the drug. For example, because of the risk of anaphylaxis, iron dextran injection should be judiciously used in cases having severe anaemia not responding to oral therapy.
Potential grave adverse events that may be prevented or intensity can be minimised by appropriate prescribing. This can be done by keeping patient under surveillance (e.g., liver function tests for valproic acid) or judicious case selection (e.g., avoidance of angiotensin-converting enzyme [ACE] inhibitors in pregnancy).
Mandatory restrictions to ensure safe use. For example, in the USA, physicians must complete a certification programme before prescribing isotretinoin. Other drugs, such as chemotherapeutic agents, may be administered only in supervised or inpatient settings.[3,4]
BW AND DRUGS
According to the USFDA’s official website, 462 drugs were issued BW from January 1, 2015, to January 31, 2024. Two hundred and twenty-five out of 462 BWs were further analysed in a longitudinal analysis.[5] Of 225 BW analysed, 65 were newly added, the revision was done in 151 and 9 were deleted from the list. This was done based on post-marketing studies in 78% of BW, pre-marketing studies in 19% of BW and animal studies in 2% of BW.
Going drug class wise, anticancer/immunosuppressants are the most common class for which BW is issued. This is followed by antimicrobials [Figure 1].[5]
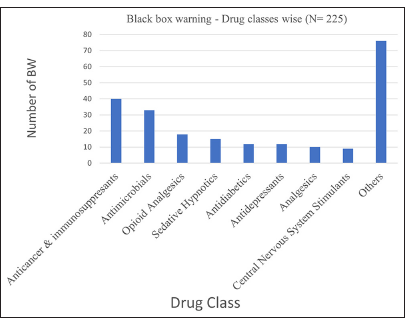
- Boxed warning (BW) - drug classes wise (n=225).
The most common BW is related to drug addiction followed by drug hypersensitivity reactions [Figure 2].[5]
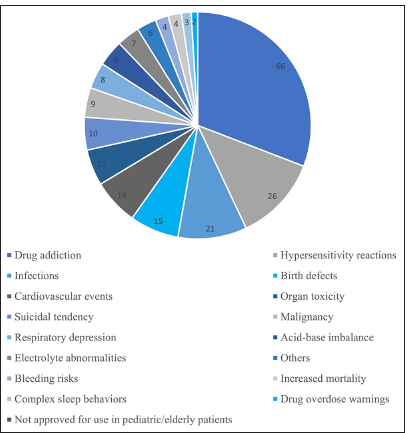
- Types of boxed warnings.
BWs related to some commonly prescribed drugs have been enlisted in Table 1.[5-9]
| Drug | Summary of boxed warning |
|---|---|
| Abacavir sulphate/Lamivudine/Azidothymidine | Haematologic toxicity, hypersensitivity reactions, lactic acidosis, myopathy and severe hepatomegaly with steatosis, Exacerbations of hepatitis B |
| Acetaminophen |
|
| Alprazolam | Abuse, misuse, addiction, dependence, withdrawal reactions, risks from concomitant use with opioids |
| Aspirin | Abuse, misuse, addiction, accidental ingestion, life-threatening respiratory depression, opioid analgesic risk evaluation and mitigation strategy, neonatal opioid withdrawal syndrome |
| Bedaquiline fumarate | QT prolongation, increased mortality |
| Captopril | Foetal toxicity |
| Carbamazepine |
|
| Chlorpheniramine maleate | Risks when simultaneously used with benzodiazepines or other central nervous system depressants. Ultrarapid metabolism of codeine and other risk factors for life-threatening respiratory depression in children |
| Clonazepam | Risk when simultaneously used with opioids |
| Clopidogrel bisulphate | Patients with two loss-of-function alleles of the CYP2C1 9 gene (CYP2C1 9 poor metabolisers) have reduced antiplatelet effect. |
| Codeine phosphate | Risks when simultaneously used with benzodiazepines or other central nervous system (CNS) depressants. Ultra-rapid metabolism of codeine and other risk factors for life-threatening respiratory depression in children |
| Diclofenac sodium | Risk of gastrointestinal events and cardiovascular events |
| Ethinylestradiol | Combination with hepatitis C drug leads to hepatic enzyme elevation |
| Febuxostat | Gout cases having cardiovascular (CV) disease treated with febuxostat had a higher rate of CV death compared to those treated with allopurinol. It should be prescribed only in cases not responding or intolerant to allopurinol. |
| Furosemide | Fluid/electrolyte loss |
| Glipizide/Metformin hydrochloride | Lactic acidosis |
| Ibuprofen/Famotidine | Serious gastrointestinal bleeding, ulceration and perforation, serious cardiovascular thrombotic events |
| Iron Dextran | Anaphylactic-type reactions, Appropriate use |
| Isoniazid/Rifampicin | Hepatotoxicity |
| Levonorgestrel | Combination with hepatitis C drug leads to hepatic enzyme elevation. |
| Levothyroxine sodium | Not for the treatment of weight loss. |
| Lorazepam | Risks when simultaneously used with opioids |
| Montelukast sodium | Serious neuropsychiatric events, aggression, agitation, sleep disturbances, depression, suicidal thoughts and behaviour |
| Morphine sulphate/Naltrexone hydrochloride | Accidental ingestion, interaction with alcohol, addiction, abuse and misuse, opioid analgesic risk evaluation and mitigation strategy, life-threatening respiratory depression, neonatal opioid withdrawal syndrome, risks when simultaneously used with benzodiazepines |
| Naproxen sodium | Serious gastrointestinal bleeding, ulceration and perforation, serious cardiovascular thrombotic events |
| Phenytoin sodium | Cardiovascular risk when rapidly infused |
| Propranolol hydrochloride | Cardiac ischaemia if abruptly discontinued |
| Tramadol hydrochloride | Addiction, abuse and misuse potential; risk of life-threatening respiratory depression |
| Zolpidem | Complex sleep behaviours such as sleep-walking and sleep-driving |
HLA: Human leucocyte antigen
BWs related to various drugs prescribed commonly in Dermatology practice have been enlisted in Table 2,[10,11] with certain important ones being discussed in detail.
| Drug | Summary of boxed warning | |
|---|---|---|
| Azathioprine |
|
|
|
Azoles
|
|
|
|
|
|
|
Botox
|
|
|
|
Calcineurin inhibitors (topical)
|
|
|
| Clindamycin | Clostridioides difficile associated diarrhoea risk | |
| Cyclosporine in psoriasis |
|
|
| Doxepin |
|
|
| Drospirenone/ethinyl estradiol |
|
|
| Fluoroquinolones ciprofloxacin, Gemifloxaci, Levofloxacin, Moxifloxacin, Norfloxacin, Ofloxacin |
|
|
| Hydroxychloro quine |
|
|
| Intravenous immunoglobulin |
|
|
| Methotrexate |
|
|
| Methoxsalen (8-methoxypsoralen) |
|
|
| Mycophenolate mofetil |
|
|
|
Retinoids
|
|
|
|
||
| Rituximab |
|
|
| Sirolimus |
|
|
| Spironolactone |
|
|
| Thalidomide | Teratogenicity:
|
|
|
Tumour necrosis factor alpha inhibitors
|
|
|
| Tofacitinib (oral) |
|
|
| Omalizumab |
|
|
ADR: Adverse drug reaction, PUVA: Psoralene plus ultraviolet A radiation, UVB: Ultraviolet B radiation
BW FOR DRUGS USED IN DERMATOLOGY PRACTICE
Topical calcineurin inhibitors (TCI)
In 2000 and 2001, the USFDA approved topical tacrolimus ointment (0.03% and 0.1%) and pimecrolimus cream (1%) respectively, as second-line agents for atopic dermatitis (AD) in children aged 2 years or above. By 2006, post-marketing surveillance revealed isolated case reports of heightened potential of lymphoma and cutaneous cancer, mainly in organ transplant recipients and in animal studies. However, in spite of no proof of a causal relationship, a BW was declared, mentioning that the long-term safety of TCIs had not been established.
As a result, less prescription generation from physician and cessation of its use by the patients were observed, ultimately leading to suboptimal control of AD.[12]
Following this, a Prospective Paediatric Longitudinal Evaluation to Assess the Long-Term Safety (APPLES) of Tacrolimus Ointment for the Treatment of AD cohort of children exposed to tacrolimus ointment for AD was conducted over a period of 10 years. It was concluded that the cancer incidence was in close proximity with an age and sex-matched control population. As per APPLES, there was a lack of proof supporting the potentially heightened risk of cancer in children treated with tacrolimus ointment for AD.[13] Regardless of FDA’s BW, till date, there are no published data about the heightened risk of cancer due to TCIs in either children or adults.[14]
As per the opinion of The Canadian Society of Allergy and Clinical Immunology (CSACI), advantages of TCIs should be judiciously assessed against the theoretical risks while prescribing. CSACI also accepts the need for long-term studies. TCIs are important steroid-sparing agents, not having any site or time restrictions and are free from atrophogenicity, and hence, they have a significant place in the management of chronic inflammatory dermatoses like AD.
The European Academy of Dermatology and Venereology position statement cites that the occurrence of Lymphoma in cases treated with TCI was no greater than in the general population.[15]
The basis of the warning is theoretical and not supported by epidemiological and clinical data. The American Academy of Dermatology thus opines that this warning is not necessary and is misleading. TCIs when used rationally are not dangerous.
Janus kinase (JAK) inhibitors
On the basis of post-marketing surveillance data on the safety of tofacitinib in cases of rheumatoid arthritis (RA), FDA placed BW related to the risk of venous thromboembolism (VTE) to tofacitinib label in 2019.[16]
Long-term safety data collected as a part of FDA-mandated post-marketing phase IIIb-IV study suggests that the risks of malignancy, VTE and major adverse cardiovascular events (MACE; cardiovascular death, nonfatal myocardial infarction and stroke) were higher with tofacitinib as against TNF inhibitor in cases having comparable baseline co-morbidities. This prompted FDA to place a BW on all approved JAK inhibitors in 2021.
Baseline risk factors such as history of VTE, age above 65 years, smoking, hypertension or coronary artery disease and hormone replacement therapy/oral contraceptive use considerably escalate the chances of VTE or MACE in cases on JAK inhibitors.[17] The limitation of studies carried out in RA cases is that RA itself is associated with risk of MACE, VTE and malignancy.[18-21]
In dermatology practice, tofacitinib is mainly prescribed for alopecia areata (AA), vitiligo and AD. The patient population is younger and has fewer co-morbidities as compared to RA cases. Other studies have also revealed that tofacitinib use in dermatologic conditions such as AA, AD, vitiligo and psoriasis was not associated with the elevated risk of VTE provided they have no high-risk factor for the same.
The punch line is that JAK inhibitors should be used only if there is a clear indication and after meticulous screening for risk factors.
Rituximab-BW
-
Infusion-related reactions (IRRs)
It can result in serious/life-threatening IRR, usually while administering the first infusion within 30– 120 min.
The manifestations and sequelae of IRR include urticaria, hypotension, angioedema, hypoxia, bronchospasm, pulmonary infiltrates, acute respiratory distress syndrome, myocardial infarction, ventricular fibrillation, cardiogenic shock, anaphylactoid events or death.
-
Severe mucocutaneous reactions with potentially fatal outcomes
These reactions include paraneoplastic pemphigus, Stevens–Johnson syndrome, lichenoid dermatitis, vesiculobullous dermatitis and toxic epidermal necrolysis.
Such reactions are observed during 1–13 weeks after infusion.
-
Progressive multifocal leukoencephalopathy (PML)
In cases having haematologic malignancies or autoimmune diseases, JC virus infection with resultant PML and death can occur. The majority of cases having haematologic malignancies and diagnosed with PML received rituximab along with other chemotherapeutic agents or as a part of haematopoietic stem cell transplant.
Most cases of PML were diagnosed within 12 months of their last infusion of rituximab.
-
Hepatitis B reactivation
It can result in fulminant hepatitis, hepatic failure and death in cases who may or may not be hepatitis B surface antigen positive.
It can also occur in cases who appear to have resolved hepatitis B infection.
It is often followed by hepatitis.
-
Infections
Grave/life-threatening bacterial, fungal and viral (new or reactivated) infections can occur while on therapy or up to 1 year after completion of therapy. Viruses such as cytomegalovirus, herpes simplex virus, parvovirus B19, varicella-zoster virus, West Nile virus and hepatitis B and C are reported to cause infections. In cases of severe infections, discontinuation of rituximab is essential.
Omalizumab
Receiving omalizumab can lead to anaphylaxis, presenting as bronchospasm, hypotension, syncope, urticaria and/or angioedema of the throat or tongue.
Anaphylaxis is reported not only after the first injection of omalizumab but it is also reported beyond 1 year after initiating the therapy.
Omalizumab therapy should be instituted in a healthcare setup having facility to manage life-threatening events like anaphylaxis.
Educate patients about the signs and symptoms of anaphylaxis and advise them to seek urgent medical care when such symptoms occur.
TAKE HOME MESSAGE
Assess the risk v/s benefits and prescribe the drug only if benefits outweigh the risks and if the disease is not controlled by the safer alternatives.
Counsel and educate the patient regarding potential side-effects and take informed consent.
Follow the baseline assessment protocol before initiating the drug in question.
Pre-medicate the patient, if recommended.
Administer the drug safely as per the guidelines.
Be fully aware of ADRs and identify them at the earliest.
Be prepared with emergency measures.
Report the ADRs to pharmacovigilance programmes.
CONCLUSION
The USFDA does not control the practice of medicine. The onus is on the prescribing physician to weigh benefit versus risk of the drug and take the decision in the best interest of the patient. BWs are not absolute contraindications for drugs, but the main purpose is to make the clinician aware about the potentially serious side effects. Nevertheless, there is no precise metric to establish when and how to apply the BW.
Multiple Choice Questions
-
Boxed warnings assigned to medications are related to:
Allergic reactions
Teratogenicity
Carcinogenicity
Most serious type of adverse events
-
What is the site of Boxed warnings on package insert?
Top
Centre
Bottom left
Bottom right
-
How many boxed warnings have been issued in the past decade by USFDA?
662
262
62
462
-
What is the basis of updating boxed warnings?
Post-marketing studies
Pre-marketing studies
Animal studies
All of the above
-
Which is most common class of medications that have been issued boxed warning?
Opioid analgesics
Anticancer and immunosuppressants
Antimicrobials
Sedative hypnotics
-
The most common boxed warning issued is related to
Drug addiction
Hypersensitivity reaction
Infections
Cardiovascular events
-
Which of the following is the boxed warning issued for topical calcineurin inhibitors?
Peripheral neuropathy
Rare malignancies
Thrombosis may occur in predisposed cases
Hypersensitivity reactions
-
Which of the following is not a boxed warning for azathioprine?
Malignancy risk
Mutagenic potential
Haematological adverse events
Congestive heart failure
-
“Clostridioides difficile associated diarrhoea risk” is a boxed warning associated with which drug?
Clindamycin
Cyclosporine
Ciprofloxacin
Calcineurin inhibitors
-
“Risk of infection from Legionella and Listeria” is boxed warning associated with which drugs?
Adalimumab
Etanercept
Infliximab
All of the above
Answer key:
1 - d, 2 - a, 3 - d, 4 - d, 5 - b, 6 - a, 7 - b, 8 - d, 9 - a, 10 - d
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
Dr. Yogesh Marfatia is on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship:
Nil.
References
- “Box Warning” In: In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023.
- [Google Scholar]
- FDA Regulation of Prescription Drugs. N Engl J Med. 2017;376:674-82.
- [CrossRef] [PubMed] [Google Scholar]
- Warnings and Precautions, Contraindications, and Boxed Warning Sections of Labeling for Human Prescription Drug and Biological Products-content and Format. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm075096.pdf [Last accessed on 2009 Nov 24]
- [Google Scholar]
- Black Box Warnings in Prescription Drug Labeling: Results of a Survey of 206 Drugs. Food Drug Law J. 1998;53:403-11.
- [Google Scholar]
- A Longitudinal Analysis of Black Box Warnings: Trends and Implications for Drug Safety. Cureus. 2024;16:e57597.
- [CrossRef] [Google Scholar]
- Black Box Warning List FADIC. 2024. Available from: https://fadic.net/black-box-warning-list [Last accessed on 2024 Dec 28]
- [Google Scholar]
- FDA Drug Safety Communication. 2014. Prescription Aceta-minophen Products to be Limited to 325 mg Dosage Unit. U.S. Food and Drug Administration; Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-prescription-acetaminophen-products-be-limited-325-mg-dosage-unit [Last accessed on 2024 Dec 28]
- [Google Scholar]
- FDA Adds Boxed Warning for Increased Risk of Death with Gout Medicine Uloric (febuxostat) 2019. U.S. Food and Drug Administration. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-increased-risk-death-gout-medicine-uloric-febuxostat [Last accessed on 2024 Dec 28]
- [Google Scholar]
- FDA Adds Boxed Warning About Risk Of Serious Injuries Caused by Sleepwalking with Certain Prescription Insomnia Drugs. 2016. U.S. Food and Drug Administration. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia [Last accessed on 2024 Dec 28]
- [Google Scholar]
- The value of the black box warning in dermatology. J Drugs Dermatol. 2015;14:660-6.
- [Google Scholar]
- Drug Labels for Tumor Necrosis Factor-Alpha (TNF-α) Blockers Now Include Warnings About Risk of Serious Infections and Cancers. 2019. U.S. Food and Drug Administration. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-drug-labels-tumor-necrosis-factor-alpha-tnfa-blockers-now-include [Last accessed on 2024 Dec 28]
- [Google Scholar]
- Cancer Risk with Topical Calcineurin Inhibitors, Pimecrolimus and Tacrolimus, for Atopic Dermatitis: A Systematic Review and Meta-analysis. Lancet Child Adolesc Health. 2023;7:13-25.
- [CrossRef] [PubMed] [Google Scholar]
- No Evidence of Increased Cancer Incidence in Children Using Topical Tacrolimus for Atopic Dermatitis. J Am Acad Dermatol. 2020;83:375-81.
- [CrossRef] [PubMed] [Google Scholar]
- CSACI Position Statement: Safety of Topical Calcineurin Inhibitors in the Management of Atopic Dermatitis in Children and Adults. Allergy Asthma Clin Immunol. 2013;9:24.
- [CrossRef] [PubMed] [Google Scholar]
- Safety of JAK Inhibitors: Focus on Cardiovascular and Thromboembolic Events. Expert Rev Clin Immunol. 2022;18:233-44.
- [CrossRef] [PubMed] [Google Scholar]
- POS0237 Major Adverse Cardiovascular Events, Malignancies and Venous Thromboembolism by Baseline Cardiovascular Risk: A post-hoc Analysis of Oral Surveillance. Ann Rheum Dis. 2022;81(Suppl 1):356-7.
- [CrossRef] [Google Scholar]
- Risk of Venous Thromboembolism in Patients with Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2013;65:1600-7.
- [CrossRef] [PubMed] [Google Scholar]
- Malignancy Incidence, Management, and Prevention in Patients with Rheumatoid Arthritis. Rheumatol Ther. 2017;4:333-47.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of Malignancy in Adult Patients with Rheumatoid Arthritis: A Meta-analysis. Arthritis Res Ther. 2015;17:212.
- [CrossRef] [PubMed] [Google Scholar]
- POS0519 Relationship between Disease Activity and Major Adverse Events in Patients with Rheumatoid Arthritis on Tofacitinib or TNF Inhibitors: A post-hoc Analysis of Oral Surveillance. Ann Rheum Dis. 2022;81(Suppl 1):517-8.
- [CrossRef] [Google Scholar]







